
diversity
Review
Deep Ecology, Biodiversity and Assisted Natural Regeneration
of European Hemiboreal Forests
Raimundas Petrokas 1,* , Diana-Abasi Ibanga 2,3,4 and Michael Manton 5
1 Department of Forest Genetics and Tree Breeding, Institute of Forestry, Lithuanian Research Centre for
Agriculture and Forestry, Liepu˛ 1, Kaunas Distr., LT-53101 Kaunas, Lithuania
2 African Ecological Memory Proj., Department of Philosophy, University of Calabar, P.M.B. 1115,
Calabar 540271, Nigeria
3 Leeds Arts and Humanities Research Institute, University of Leeds, Leeds LS2 9JT, UK
4 Leeds University Centre for African Studies, University of Leeds, Leeds LS2 9JT, UK
5 Faculty of Forest Sciences and Ecology, Vytautas Magnus University, Studentu˛ 13, Kaunas Distr.,
LT-53101 Kaunas, Lithuania
* Correspondence: raimundas.petrokas@lammc.lt
Citation: Petrokas, R.; Ibanga, D.-A.;
Manton, M. Deep Ecology,
Biodiversity and Assisted Natural
Regeneration of European
Hemiboreal Forests. Diversity 2022,
14, 892. https://doi.org/10.3390/
d14100892
Abstract: Climate change and the associated disturbances have disrupted the relative stability of
tree species composition in hemiboreal forests. The natural ecology of forest communities, including
species occurrence and composition, forest structure, and food webs, have been affected. Yet, the
hemiboreal forest zone of Lithuania is the least studied in the country for climate change risks and
possible management adaption techniques. This problem is further complicated by the fact that
Lithuania uses a traditional centralised forest management system. Therefore, this work proposes
assisted natural regeneration (ANR) of tree species as a more viable means of building hemiboreal
forest resilience to cope with future climate change risks. The ANR model implies that forest manage-
ment is localised in local communities, to provide opportunities for the local people to participate
in forest management based on local knowledge, thereby facilitating the transition from cultural
diversity to biodiversity. Further, ANR is grounded on an ethical framework—deep ecology—to
provide ethical justification for the proposal to transit forest management in Lithuania from the
traditional centralised segregated system to a community-driven practice. The work combines the
theories of ANR, deep ecology, and hemiboreal forest knowledge systems to provide complementary
information that builds on gaps in the existing literature. This study is unique in that no previous
work has linked ANR and deep ecology in the context of Lithuania’s forest ecosystems.
Academic Editors: Jian Lin, Qin Ma,
Jingyu Dai, Weichao Guo and Yang Ju
Received: 23 September 2022
Accepted: 19 October 2022
Published: 21 October 2022
Publisher’s Note: MDPI stays neutral
with regard to jurisdictional claims in
published maps and institutional affil-
iations.
Copyright: © 2022 by the authors.
Licensee MDPI, Basel, Switzerland.
This article is an open access article
distributed under the terms and
conditions of the Creative Commons
Attribution (CC BY) license (https://
creativecommons.org/licenses/by/
4.0/).
Keywords: hemiboreal trees; assisted natural regeneration; deep ecology; climate change; forest
sustainability; forest management; Lithuania; forest disturbances
1. Introduction
Natural recurring forest processes are often self-organised and implicate sustainabil-
ity processes in environmental changes. Niche construction, ecological engineering, and
biosemiotics processes are different forms of indirect, background interaction and commu-
nication of organisms in the environment [1]. Self-organisation of an ecosystem includes all
the diversity that cannot be reduced to the properties of an individual system’s components,
such as molecules, genes, populations, and species in both time and space [2]. Meaning is
generated across all the organisational levels [3]. The strategy of life expansion is realized
through the spread of life in space—the proliferation and collaborative construction of
ecosystems and the biosphere by organisms. Forests are multi-scale, multi-species net-
works that constantly evolve toward the successional processes and patterns of natural
regeneration which cannot be reached at an individual tree species level. In this direction,
our work focuses on the super-organism approach of forest communities that considers
Diversity 2022, 14, 892. https://doi.org/10.3390/d14100892
https://www.mdpi.com/journal/diversity

Diversity 2022, 14, 892
2 of 11
succession as a comprehensive ecological process of multiple events where the forest vege-
tation communities are directly related to environmental condition with regard to climate
change [4].
The life history traits of species are controlled by natural patterns and processes
recurring over time and space at multiple scales [5,6]. Natural selection has matched trees
to site and environmental conditions for millennia [7] and is considered a key evolutionary
process that can increase the adaptation rate of species to environmental change [8]. Natural
selection can be confirmed through field observations of ecological communities and
their development towards self-organisation. Tree species’ life histories, reproductive
character, regeneration times, mode of dispersion, and other evolutionary phenomena are
interconnected in the immense and complex system of self-sustaining interactions of forest
communities [9]. The ecology of a forest never ceases to evolve. The probability of seed
germination, tree growth, development and recruitment is dependent on a species’ genetic
profiles and life history traits to cope with the changes in environmental conditions [10–12].
Dynamics in forest communities are driven by a wide range of factors, including species’
invariable life history strategies [4].
However, traditional forest management, climate change and increased disturbances
have disrupted the relative stability of tree species composition in combination with the
edaphic site conditions in European hemiboreal forests [13,14]. This is a key problem.
The natural ecology of forest communities, including species occurrence and composition,
forest structure, and food webs have been affected [15]. Developing knowledge about
natural forest disturbance dynamics and their relationship to anthropogenic impacts and
management practices is essential towards the mitigation of impacts on forest ecosystems
in the light of climate change [16]. This warrants the basis for proposing the assisted natural
regeneration (ANR) strategy as an alternative adaptive model for forest management
in Europe.
The aim of the review was to provide complementary information that builds on the
topics of deep ecology and ANR within the context of hemiboreal forest management. First,
we proposed a conceptual framework for hemiboreal tree dynamics based on Lithuania
as a case. Second, we discussed the benefits of ANR of trees in the context of hemiboreal
ecology. Finally, ANR was overlaid on deep ecology—an ethical framework—to highlight
how it can promote diversity in forest management.
2. European Hemiboreal Tree Species: The Case of Lithuania
We focused on the European hemiboreal forests of Lithuania because it is one of only
two countries (Latvia and Lithuania) that falls completely within the hemiboreal forest zone
in Europe [17]. The hemiboreal forest zone is the flux zone between the temperate forest
zone to the south and boreal zone to the north. Unfortunately, the forests of the hemiboreal
zone are often overlooked in climate impact and adaption studies, while attention is focused
on the other two zones [14].
Lithuania’s hemiboreal forest site types (as well as the 13 Natura 2000 forest habitat
types of European Community) can be classified into three main forest habitat types based
on the concept of potential vegetation and soils [14,18,19]: (1) mixed broadleaved forests
on rich sites; (2) mixed species forests on mesic sites dominated by Norway spruce; and
(3) Scots pine (Pinus sylvestris) forests on poor sites. Soil moisture and fertility of Lithuania’s
forests are considered the main drivers of forest disturbances and succession [17]. As such,
Lithuania’s forests have been classified by the Food and Agriculture Organisation (FAO,
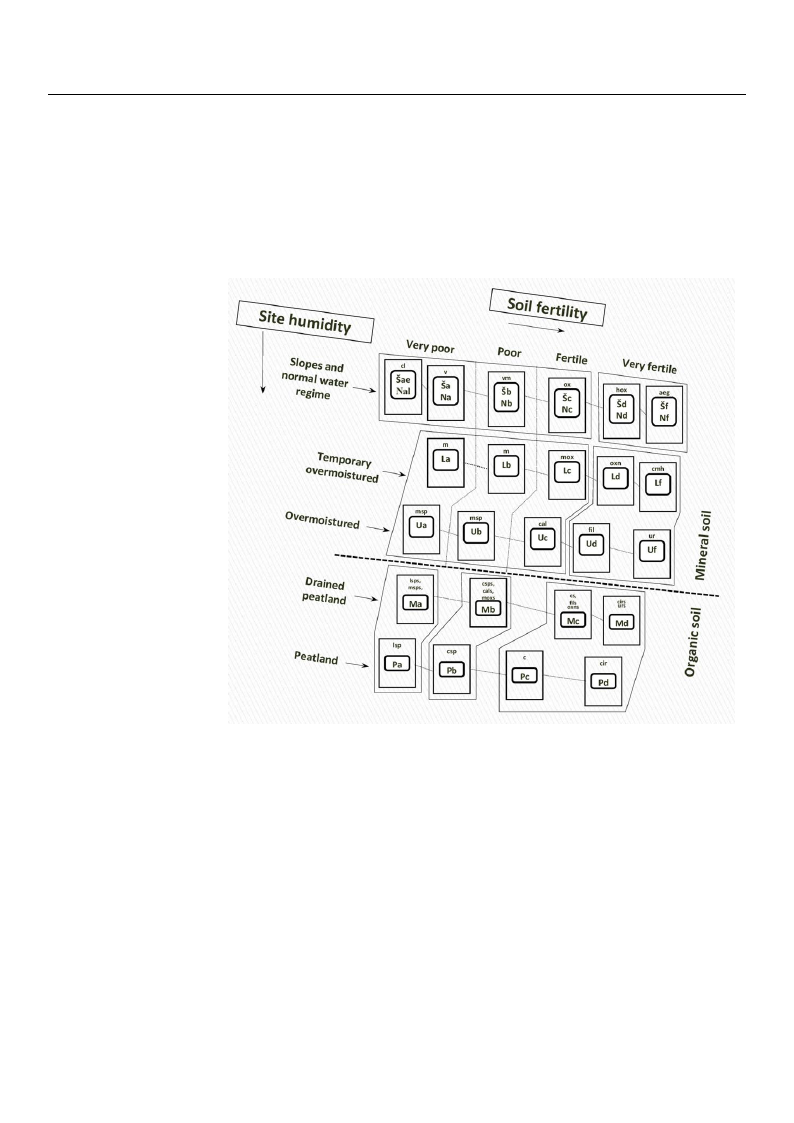
Rome, Italy) soil classification system [20,21] based on soil typological groups (Figure 1).
The main tree species of mixed broadleaved forests in Lithuania are Quercus robur,
Tilia cordata, Acer platanoides, Fraxinus excelsior, and Ulmus glabra, along with Alnus incana
and Alnus glutinosa [18]. Other individual non-dominant tree species can also be found
here—Norway spruce and birch are the most common, the least common being Scots
pine [22]. Mixed Norway spruce forests in Lithuania usually consist of Betula pendula, less
commonly Populus tremula or Pinus sylvestris, and on richer sites Quercus robur, Tilia cordata,
Diversity 2022, 14, x FOR PEER REVIEW
Diversity 2022, 14, 892
3o
3 of 11
commonly Populus tremula or Pinus sylvestris, and on richer sites Quercus robur, Tilia c
Acer platanoides daantda, CAacreprinpulastabneotiudleussa. nEdurCaasripainnuasspbeetnulauns.dEbuirracshiasntaansdpsenaraenmdobsitrlcyhmstiaxnedds, are mos
as well as Englimshixoeadk, aasndweElul aros pEenagnlisahshoasktaanndds E[2u2r]o.pSecaontsasphinsteafnodrses[2ts2]g. rSocwotsopninheigfholryests grow
oligotrophic, strhoingghllyy aocliigdo-trtoopbhaisce, -srtircohngsolyilasc, iodn- tvoerbyasseh-railclhowsoailns,dodnrvyersyubshstarlalotews atondwderty substra
and oxygen-pootromwireetsa, nodn moxiyngeernal-paonodr pmeiartews, eotnlanmdins.erWalitahnind ppeeaattlawnedtlfaonrdess.tsW, vitehgientapteioantland fore
communities shvoewgettahtaiot na choimghm-uwnaitteierstsahbolwe athnadt nauhtirgihe-nwt apteorotrabenlevairnodnnmuetrnitenatffpeocotsr etrneveironment
growth [17]. Thfeecstspetrceieesgrcoowmtpho[s1i7t]io. TnhoefsLpietchiuesancoiamnphoesimtioibnoorfeaLlitphiunaenifaonrehsetsmiisboorfetaelnpaine forest
mixture of specioefstefnroammvixatruiroeuosfvspegeceiteastiforonmfovramriaotuios nvsegbeutattcioann fboermreamtioanrks abbultycasinmbielarremtoarkably s
boreal pine foresiltasr(etospbeocrieaalllypionne ifnofrerstisle(essiptesc)ia. lly on infertile sites).
Figure 1. LithuanbFaiigasu’esdrefoo1nr.eLssoittihlsuifteaernttiialyi’tpsyefaosnrcedhstemmsioteies.ttuyTrpehe,esacnbhdoelmtdheec. osTmdheeaslbl ronelodfencr-obtdooeldsLriletehtfteuerrastnoriaLenfiet’hrssufatoonrietahsnet’ssfoiftoreereststvseigteettayt
types based on sotyilpfeersteirliietys [a1n8,d20m,2o3]is: tNu—ren, oarnmdatlhlyemsmoisatl,lLn—onte-mboplodralertiltyerosvreerfmerosistto, Uth—e ofovreersmt oviesgt,-P—peatla
etation type seriesan[1d8f,—20v,2e3ry]: eNut—ronpohricmsaolilys, md—oiesut,trLo—phtiecmsopiolsr,acr—ilymoevsoetrrompohiscts,oUil—s, bo—veorlimgootirsotp, hPi—c soils, a—v
peatland, and f—ovleigroytreoupthroicphsoicils;oialse,gd——Aeguotproodpiohsiac, sco—ilsC,acri—comsa,escoatlr—opChaliacmsaogirloss,tbid—osao,ligciort—roCpahriicco-iridosa, c
soils, a—very oligColtardoopnihoisca,scomilhs;—aCegar—icAo-emgioxptoohdeirobsoas,ac,—cspC—arCicaorsiac,o-csaplh—agCnaolsaam, failg—roFsitliidpoensad,ucloir-m—ixCtaorhiecrob-osa, hox—
iridosa, cl—Cladonipoastaic,oc-moxha—lidCosaar,iclsop-m—ixLteodhoe-rsbpohsaag,ncosspa,—mC—arMicoy-rstpilhloasgan, omsao,xfi—l—MFyirltipilelon-douxaloli-dmoisxat,omhesrpb—osaM, yrtillo-sph
hox—Hepatico-oxanliodsoas,ao,xls—pO—xLaeliddoo-ssap,hoaxgnn—osOa,xmal—idoM-nyermtiolrloossaa,, umro—xU—rMticyosrati,lvlo—-oVxaalcicdionsiao,sam, vspm——MVaycrctiinllioo--myrtillosa
sphagnosa, ox—Ox3al.idHoseam, oibxno—reOalxaTlirdeoe-nDemynoraomsa,icusr—Urticosa, v—Vacciniosa, vm—Vaccinio-myrtillosa.
3. Hemiboreal T3r.e1e. TDreyenRaemgeincesration Strategy
3.1. Tree RegenerationFSotrreastetsgyare characterized by the development of contiguous communities of trees t
ttihaaltaaFrrroearnergsetelsamtaievreneatmrtlicay,rphuensluagniertreineetsamliicaqcefaotetsuilenrvecarmtrseil,elziyitsaesiyitntd,ueeodcncnbqoiobefyunomyadorthlmphfiituteoitymohis,ndnieacte,iocnmovoaninemnodnl,sdtoipettspiorltodovrmsnuiiecst,enacitaonitttnuiinntoogdr,nnouesfl,i[ttos2roachuc4ogaic–dnnett2iiug,tos6irtsn]gfei.ienu,zTtaogeoaht,guuuedecirsais,elsbahcstssisoineztosmhgne,f,eucdmmemciislsuooahtffdnsrrstsiiioh,tfbtrimiedueuemdistcsiattoouffdronrifrjobraa,teumlcrssteleptieneaosagsdnt-asj,cuaiscbepejseanacttittaecmldo
communities created by human intervention [24–26]. The absence of structural legacies
at multiple scales is one of the most distinguishing features of modified forests subjected
to intense and frequent anthropogenic disturbances [27,28]. Species’ life history traits are
interrelated with natural disturbances and associated site conditions, and these account for

Diversity 2022, 14, 892
4 of 11
the interactions (patterns and processes) in species distribution [14,29]. There is also increas-
ing evidence that the intrinsic influences of disturbance susceptibility are phylogenetically
inherited, implying that species-level traits are constrained by developmental, genetic, or
other correlated limitations [30]. Being the primary species of forest ecosystems, long-lived
trees are pivotal in providing associated organisms with a combination of resources and
habitats that range from beneficial to detrimental [31]. Therefore, the forest development
and growth dynamics of tree species follow relatively fixed patterns and can be difficult to
modify in the light of the interactions of both biological and physical processes. This is also
the case with hemiboreal trees’ natural regeneration.
There are four tree natural regeneration strategies, i.e., the establishment and growth of
trees in forest gaps [32–36]: (i) colonization; (ii) occupation; (iii) invasion; and (iv) expansion
(Table 1). These are inter-intuitive with Clark and Clark’s [37] tree species regeneration
groups (A–D), Whitmore’s [38] tree species groups, having an increasing “pioneer index”
(1–4), and Grime’s [39] four types of secondary ecological strategies in trees that are derived
from the theoretical triangular scheme of competitor (C), stress-tolerant (S) and ruderal
(R) primary plant ecological strategies—stress-tolerant ruderals (S-R), competitive stress-
tolerant ruderals (C-S-R), competitive ruderals (C-R), and competitive stress-tolerators
(C-S). Colonization (D, 4, S-R) implies that even-aged seedlings are being established
after gap formation and grow only in gaps. This relates to stress-tolerant species that
possess a ruderal strategy without advanced regeneration. Juveniles have the highest
growth potential. A ruderal strategy is a characteristic of many species that never become
established in ruderal habitats. Ruderal species are plants that grow only in habitats that
have been completely disturbed and damaged by human activity [40]. Occupation (C,
3, C-S-R) relates to the competitive stress-tolerant ruderal strategy species occurring as gap
makers. Their seeds germinate better in gaps with intermediate canopy openness than in
the understorey or large gaps, saplings can survive in closed forests. Invasion (B, 2, C-R)
implies that trees regenerate from saplings recruited before gap or stand formation. This
type involves competitive species with a ruderal strategy of advance regeneration, allowing
already established juveniles to survive in newly created gaps. Expansion (A, 1, C-S)
implies that trees in the forest regenerate as advanced regeneration under shade. This
usually involve competitive stress-tolerant species. Juveniles have average growth rates.
3.2. Natural Regeneration of European Hemiboreal Tree Species
Multiscale recovery dynamics analysis of community typology is measured to deter-
mine the impact of change in forest ecology. Usually, it ranges from tree genetic variation
characteristics (in terms of regeneration vs. canopy compositions) to multi-population
structures reflected in disturbance and management regimes. To enhance the adaptive
potential and associated ecosystem services of forests, we proposed a conceptual frame-
work for hemiboreal tree dynamics based on a dynamic typology of forest communi-
ties [13,14,18,19,41–46] and forest sites defined by field layer-canopy dominants, on-site soil
fertility and moisture [18,47], and four types of tree regeneration strategies [33–35,37–39,48]
(Table 1). It follows the Lithuanian classification of forest types and the layer dominants:
forest site type, forest type series (field flora), and dominant and secondary tree species [18].
The three dynamic forest habitat types in our conceptual framework represent general
descriptions of plant community types that reflect the dynamics of vegetation cover that
occur in the course of natural disturbances [13]. In hemiboreal forests, there are three main
types of natural disturbance regimes that determine the success of natural selection: (1) gap
dynamics caused by the death of individual trees or small groups of trees in the absence
of fire; (2) successional development after severe stand-replacing disturbances, such as
crown fires, large windthrows, pest outbreaks, etc.; and (3) multi-cohort dynamics related
to partial disturbances, such as low-intensity surface fires [41–46].
Hemiboreal forests may be legacies of biological and physical disturbances [6,24].
Disturbance regimes are classified by the type, magnitude and duration of environmental
variation as well as community (ecosystem) and individual species resilience [42–44,49].

Diversity 2022, 14, 892
5 of 11
Regeneration Strategy:
C—Colonization
O—Occupation
I—Invasion
E—Expansion
C
Alnus glutinosa
C
Alnus incana
C
Betula pendula
C
Betula pubescens
C
Larix decidua
C
Pinus sylvestris
O
Fraxinus excelsior
O
Populus tremula
O
Quercus robur
O
Ulmus laevis
I
Acer platanoides
I
Carpinus betulus
I
Picea abies
I
Ulmus glabra
E
Fagus sylvatica
E
Tilia cordata
Tree species regeneration in hemiboreal zone is generally rapid after large-scale short-term
disturbances (e.g., forest fire) but slower after longer term disturbances such as repeated
logging or forest conversion to monoculture plantations [48]. Restoration of the original
forest ecosystem via natural regeneration can take several centuries as succession begins
with early-successional herb, shrub, and tree species, and culminates with late-successional
species. In order to understand forest regeneration processes following a disturbance, one
needs to be knowledgeable in forest dynamic typology, which can provide a first insight
into the status of vegetation cover (i.e., basal, canopy, foliar, or ground cover) and warn us
if it is facing decline or an unwanted trajectory. As such, we have allocated each hemiboreal
tree species to a dominant regeneration strategy (Table 1).
Table 1. Conceptual framework of hemiboreal tree dynamics in Lithuania [32,48,50]. Capital letters
indicate the main tree species that form forest stands in gap dynamics (G), successional development
(S), or multi-cohort succession (M), whereas small letters (g, s, and m) indicate secondary ones which
are a valuable admixture in these stands.
Forest Habitat Types (NATURA 2000 Codes)/Codes of the Lithuanian Forest Type Series * and Forest Site Types **
Mixed Broadleaved Forests
Norway Spruce Mixed Forests
Scots Pine Forests
(9020 9080 91F0 91E0)
(9050 9160 9180 9190 9070)
(9010 9060 91D0 91T0)
aeg * cmh cal Fil ur cir
c
ox mox hox oxn cl
v vm m msp csp lsp
Nf ** Lf Uc Ud Uf Pd Pc Nc Lc Nd Ld Nal Na Nb Lb Ub Pb Pa
G
G
G
G
G, G,
S
S
G
G
G
g
G
s, g s, g G G
G
G
g
G
g
S
S
S
S
s
s
S
G
G
S
S
g
G
S
s
S
S
s
s,
m
M
M
MMMMMMM
G
G
Gg
G
G
G
G
S
S
s
S
S
G
G
G
Gg
G
G
g
G
g
G
G
gG
S
S
S
S
s
s
s
G
G
G
G
gGg
* Field layer codes of the main types of forest plant communities, i.e., forest type series (forest site types): aeg—
Aegopodiosa (Nf), c—Caricosa (Pc), cal—Calamagrostidosa (Uc), cir—Carico-iridosa (Pd), cl—Cladoniosa (Nal), cmh—
Carico-mixtoherbosa (Lf), csp—Carico-sphagnosa (Pb), fil—Filipendulo-mixtoherbosa (Ud), hox—Hepatico-oxalidosa
(Nd), lsp—Ledo-sphagnosa (Pa), m—Myrtillosa (Lb), mox—Myrtillo-oxalidosa (Lc), msp—Myrtillo-sphagnosa (Ub),
ox—Oxalidosa (Nc), oxn—Oxalido-nemorosa (Ld), ur—Urticosa (Uf), v—Vacciniosa (Na), vm—Vaccinio-myrtillosa
(Nb). ** N—normally moist, L—temporarily overmoistured, U—overmoistured, P—peatland, and f—very
eutrophic soils, d—eutrophic soils, c—mesotrophic soils, b—oligotrophic soils, a—very oligotrophic soils (see
Figure 1).
However, a forest stand that is subject to a larger-scale disturbance can also be subject
to smaller-scale disturbance thus the scale of disturbance is also a factor that needs be
considered and discussed. For instance, in mixed Norway spruce forest, disturbance can
range from a single tree (gap/small patch (G)) to a stand or forest (large patch (S)) sized
disturbance. Thus, there is no certain rule on regeneration. Another factor to consider is the
type of disturbance. For instance, Scots pine is fire tolerant, and fire stimulates regeneration.
Conversely, Norway spruce is fire intolerant and thus is often eliminated together with
its seed bank. So, fire creates multi-cohort pine stands and eliminates spruce. Also, the
different disturbance regimes of forests undergo generate different age profiles [27]. For
instance, successional development (S) of spruce-dominated mixed forests varies across

Diversity 2022, 14, 892
6 of 11
all age classes. Multi-cohort stand succession (M) generally has older age classes mixed
with some younger age classes [27]. Another important aspect is that natural forests have
multiple ages structures due to the different regeneration modes of each tree species. For
instance, birch and aspen are pioneer species with fast regeneration but spruce is much
slower and needs time to be invasive and form the dominant stand species in a mixed
forest ecosystem.
4. Concept of Assisted Natural Regeneration of Trees
The regimes and dynamics of forest disturbances are forecast and shown to be altered
significantly by the impact of climate change [51]. Vegetation models show us that climate
change is of such magnitude and speed that tree species currently present in our forests will
not have time to adapt or acclimatize to predicted climatic conditions [52]. By the end of
the century, the native tree species that currently populate Lithuania’s forests may succumb
to climate change under traditional forest management practices and the species that may
be able to adapt to the future conditions may not have had time to naturally evolve and
adjust. This would stifle the current biodiversity. This highlights the need for knowledge
and development of sustainable forest management that imitates the natural patterns and
processes of forest ecosystems, and the development of a conceptual framework to mitigate
the barriers to natural regeneration under a changing environment.
In the context of global climate change and disturbances, the adaptive potential of
forests lies in assisted natural regeneration (ANR) in the contiguous communities of trees.
It ensures that the physical and biological conditions of the forest ecosystem can continue
to self-regulate while supporting natural selection and native biodiversity [48]. ANR is
applied to forest resources after harvesting with the aim to accelerate, rather than replace,
natural successional processes by removing or reducing barriers to regeneration such as
soil degradation, competition with weedy species, and recurring disturbances (e.g., fire,
grazing and wood harvesting) [53]. Therefore, we call for the application of ANR in the
adaptative management of forest disturbances and succession to sustain tree species and
promote forest self-organisation. ANR is very relevant in the context of hemiboreal forests.
It lays the groundwork necessary to consider the life-cycle features of trees that affect the
organic relationships between individual species and ecological communities indirectly
via their effects on growth, reproduction, and survival, such as tree regeneration strategies
that correspond to the various trade-offs in the adaptations to competition, stress, and
disturbance [54,55]. The self-organisation of an ecological community is a highly ordered
non-random process based on information written in the genomes of participating species
and it can play a major role in providing resilience to future climate change [56–58] but
must be accelerated with ANR strategies.
ANR works where local people intervene to help local plants and wildlife naturally
recover, leaning on their knowledge of native habitats and on ancestral traditions [59]. The
practice of ANR is not centralised to a given global hegemonic knowledge but varies from
place to place. Despite this, the most fundamental practices of ANR are protecting and
facilitating the growth of parent trees inherently present in the area and their regeneration,
and inextricably linked to natural disturbance regimes and site conditions [60]. The empha-
sis is on sustainability of diversity in the tree community. In fact, many studies indicate
that ANR enhances tree growth, biodiversity, and forest productivity [61]. Also, there is a
likelihood that ANR will contribute to carbon sequestration vis-à-vis slowing or mitigating
the effects of climate change [62]. The fact is that the adverse effects of the phenomenon of
global climate change can undermine the resilience of forest ecology in terms of its capacity
for natural regeneration to occur successfully on the scale of the expected time. In other
words, climate change can slow the reproductive regime of the European hemiboreal tree
species, leading to delays in the turnover of tree populations and to sustain their resilience.
Therefore, with the concept of ANR, the natural powers of forest ecology in Lithuania can
be revitalized and fast-tracked to keep up with the pace of global climate change or even
overtake it ahead its disruptions.

Diversity 2022, 14, 892
7 of 11
5. Grounding Assisted Natural Regeneration on Ethical Framework
Assisted natural regeneration (ANR) encourages non-anthropocentric intervention
in the forest ecology. This makes it necessary to anchor it on a sound ethical foundation
to guide the human–environment interactions anticipated in the model. Deep ecology is
an ethical framework that provides excellent guidelines for non-anthropocentric human-
environment interaction on the scale that can support the ANR programme. It provides
a set of ethical principles complementary with the institutional attributes of ANR, which
urges for deliberate and non-anthropocentric intervention in the ecology of trees to fast-
track it against the growing threats of global climate change. Deep ecology provides an
ethical grounding for human beings to participate in nature and explore forest ecosystems
by sharing in their pleasures and challenges, benefits and needs, and awakening a persons’
ecological consciousness [63]. Ecological consciousness presupposes humans’ freedom
of action in their organic relationships with the earth and with the plants and animals
that grow on it. Participation in forest ecology must be based on the recognition of the
intrinsic worth of tree species and other nonhuman components in the natural community.
Deep ecology places an emphasis on the recognition of intrinsic worth in trees and in the
nurturing of diversity in the management of the ecosphere [64,65].
The idea of the intrinsic worth of trees is formulated to scale down on anthropocentric
interference in the natural community. Anthropocentric interference has dominated the
history of human–environment relations, resulting in large-scale environmental degra-
dation, climate change-related global warming, and biodiversity depletion [66]. Human
beings placed themselves at the centre of nature to the neglect of the interests of nonhuman
beings—in the belief that the environment and its resources (trees, animals, minerals, etc.)
do not have value beyond the human interests they serve [63,64]. This trend in human
thinking negatively impacted the biological and ecological diversity of the natural commu-
nity [66]. So, the emphasis placed on the recognition of the intrinsic worth of nonhuman
nature (trees, animals, ecosystems, etc.) is aimed at restoring the richness and diversity of
life forms [63,64]. Diversity—biological, ecological, cultural, and cognitive diversities—is at
the heart of the deep ecology. It is recognized as the necessary ingredient that contributes to
the sustainability and flourishing of the ecosphere [65]. But the recognition of the intrinsic
worth of trees does not annul human interest in nature. Deep ecology recognizes that
human needs must be satisfied in the context of the environmental resources [63–65]. In
meeting their vital needs, human beings must replenish the environment in terms of taking
active and deliberate steps to restore it to its richness and diversity to ensure its continued
survival and flourishing [63].
The philosophy of deep ecology places emphasis on diversity as the bastion of sustain-
ability. This is inter-intuitive with the ANR model in forest management. ANR emphasizes
human intervention in forest ecology based on the local knowledge of the people [59]. In
other words, ANR encourages ecological and cultural diversity. Epistemic diversity is an
integral part of the effort to restore, preserve and sustain diversity in forest ecology via
ANR. The emphasis on epistemic diversity is based on the understanding that ‘many of the
paths to [ecological] stabilisation run straight through our daily lives’ [67]. Further, cultural
communities have embodied diverse historical knowledge about their environments and
will readily act within these contexts [65]. In this direction, while ANR embraces local
knowledge in the management of forest ecology, deep ecology recognizes epistemological
pluralism as a major ingredient that contribute to survival and flourishing of the biodiver-
sity. In line with this view, Lithuania must shift away from centralized management of its
forests while putting more responsibilities in the hands of individuals and communities—to
harness the cognitive diversity of Lithuanians to revitalize and reposition the forest ecology
ahead of the increasing negative impacts of global climate change. This does not mean that
scientific knowledge should be jettisoned. Science is crucial to understanding the genome of
trees, make quantifiable projections of the natural regeneration processes, support commu-
nities to ground strategies in evidence, and adapt the ecosystems to environmental change
via technology [66,67]. Yet, in talking about science, the hegemonic knowledge of North

Diversity 2022, 14, 892
8 of 11
America and western Europe is often promoted without regards to differences in cultural
contexts in the process, marginalising other ways of knowing and limiting opportunities
for inputs from local knowledge systems. In making allowance for collaborations between
core scientific and local knowledges, ANR, consistent with deep ecology, facilitates the
transition from epistemic and cultural diversities to biodiversity.
6. Concluding Remarks
This paper discussed the inadequacy of natural regeneration of tree species in the
context of climate change. We observed that the environmental disturbances associated
with climate change are monumental, very disruptive and that the evolution of certain
tree species will not be able to adapt in time with climate change, especially in the hemi-
boreal context of Lithuania. Hence, we argue that ANR of tree species in the hemiboreal
forest ecology should be applied to keep the forest resilient in the face of climate change.
Importantly, ANR must be based on the idea of diversity. This implies that the ANR
processes must be localised in community-based knowledge systems, which necessitates
the introduction of the concept of deep ecology to demonstrate this importance. Deep
ecology also provides an ethical justification for the proposal to transit forest management
in Lithuania from the traditional centralised system to a community-driven practice. ANR,
embedded in the principle of deep ecology, is very relevant in the context of hemiboreal
Lithuania where there is an increasing need to maintain forest biodiversity, while at the
same time enhancing the cultural diversity of Lithuania. Adopting ANR will not only
promote local participation in forest management in Lithuania but will make the forests
resilient to future climate change.
Author Contributions: Conceptualization, R.P., D.-A.I. and M.M.; writing—original draft prepara-
tion, R.P., D.-A.I. and M.M.; writing—review and editing, D.-A.I., M.M. and R.P. All authors have
read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Acknowledgments: The review presents research findings that have been obtained through the long-
term research programme “Sustainable forestry and global changes”, implemented by the Lithuanian
Research Centre for Agriculture and Forestry.
Conflicts of Interest: The authors declare no conflict of interest.
References
1. Olff, H.; Alonso, D.; Berg, M.P.; Eriksson, B.K.; Loreau, M.; Piersma, T.; Rooney, N. Parallel Ecological Networks in Ecosystems.
Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1755–1779. [CrossRef] [PubMed]
2. Camazine, S.; Deneubourg, J.-L.; Franks, N.R.; Sneyd, J.; Theraula, G.; Bonabeau, E. Self-Organization in Biological Systems;
Princeton University Press: Princeton, NJ, USA, 2020; ISBN 978-0-691-21292-0.
3. Kazansky, A.B. Bootstrapping of Life through Holonomy and Self-Modification. In Proceedings of the 9th International Conference
on Computing Anticipatory Systems, Liege, Belgium, 3–8 August 2009; Dubois, D.M., Ed.; American Institute of Physics: Melville,
NY, USA, 2010; pp. 297–306.
4. Emery, S.M. Succession: A Closer Look. Nat. Educ. 2010, 3, 10–45.
5. Schwarz, C.; Gourgue, O.; van Belzen, J.; Zhu, Z.; Bouma, T.J.; van de Koppel, J.; Ruessink, G.; Claude, N.; Temmerman,
S. Self-Organization of a Biogeomorphic Landscape Controlled by Plant Life-History Traits. Nat. Geosci. 2018, 11, 672–677.
[CrossRef]
6. Turner, M.G. Landscape Ecology: The Effect of Pattern on Process. Annu. Rev. Ecol. Syst. 1989, 20, 171–197. [CrossRef]
7. Minore, D.; Laacke, R.J. Natural Regeneration. In Reforestation Practices in Southwest Oregon and Northern California: Chapter 11;
Hobbs, S.D., Tesch, S.D., Owston, P.W., Stewart, R.E., Tappeiner, J.C., Wells, G.E., Eds.; Oregon State University Press: Corvallis,
OR, USA, 1992; pp. 258–283. ISBN 0-87437-001-9.
8. Bajc, M.; Aravanopoulos, F.; Westergren, M.; Fussi, B.; Kavaliauskas, D.; Alizoti, P.; Kiourtsis, F.; Kraigher, H. Manual for Forest
Genetic Monitoring; Slovenian Forestry Institute: Ljubljana, Slovenia, 2020.

Diversity 2022, 14, 892
9 of 11
9. Holbrook, M. Adventures in Complexity: An Essay on Dynamic Open Complex Adaptive Systems, Butterfly Effects, Self-
Organizing Order, Coevolution, the Ecological Perspective, Fitness Landscapes, Market Spaces, Emergent Beauty at the Edge of
Chaos, and All That Jazz. Acad. Mark. Sci. Rev. 2003, 2003, 1–181.
10. Borman, M.M.; Pyke, D.A. Successional Theory and the Desired Plant Community Approach. Rangelands 1994, 16, 82–84.
11. Chazdon, R.L. Second Growth: The Promise of Tropical Forest Regeneration in an Age of Deforestation; University of Chicago Press:
Chicago, IL, USA, 2014; ISBN 978-0-226-11791-1.
12. Chazdon, R.L.; Brancalion, P.H.S.; Laestadius, L.; Bennett-Curry, A.; Buckingham, K.; Kumar, C.; Moll-Rocek, J.; Vieira, I.C.G.;
Wilson, S.J. When Is a Forest a Forest? Forest Concepts and Definitions in the Era of Forest and Landscape Restoration. Ambio
2016, 45, 538–550. [CrossRef] [PubMed]
13. Ivanova, N.; Fomin, V.; Kusbach, A. Experience of Forest Ecological Classification in Assessment of Vegetation Dynamics.
Sustainability 2022, 14, 3384. [CrossRef]
14. Jõgiste, K.; Frelich, L.E.; Laarmann, D.; Vodde, F.; Baders, E.; Donis, J.; Jansons, A.; Kangur, A.; Korjus, H.; Köster, K.; et al.
Imprints of Management History on Hemiboreal Forest Ecosystems in the Baltic States. Ecosphere 2018, 9, e02503. [CrossRef]
15. Angelstam, P.; Manton, M.; Pedersen, S.; Elbakidze, M. Disrupted Trophic Interactions Affect Recruitment of Boreal Deciduous
and Coniferous Trees in Northern Europe. Ecol. Appl. 2017, 27, 1108–1123. [CrossRef]
16. European Commission. New EU Forest Strategy for 2030. In Communication from the Commission to the European Parliament, the Council,
the European Economic and Social Committee and the Committee of the Regions; European Union: Brussels, Belgium, 2021; p. 28.
17. Manton, M.; Ruffner, C.; Kibirkštis, G.; Brazaitis, G.; Marozas, V.; Pukiene˙ , R.; Makrickiene, E.; Angelstam, P. Fire Occurrence in
Hemi-Boreal Forests: Exploring Natural and Cultural Scots Pine Fire Regimes Using Dendrochronology in Lithuania. Land 2022,
11, 260. [CrossRef]
18. Karazija, S. Forest Types of Lithuania; Mokslas: Vilnius, Lietuva, 1988; ISBN 978-5-420-00421-0.
19. Brazaitis, G.; Marozas, V.; Augutis, D.; Preikša, Ž.; Šaudyte˙ -Manton, S. Lithuanian Forest Habitat Management Recommendations–
“Guidelines for the Management of Natural Forest Habitat Types of EC Importance”; Naturalit: Vilnius, Lietuva, 2021.
20. Vaicˇys, M.; Mažvila, J. The Influence of Soil Characteristics on Plant Productivity and Ecological Stability. Ekologija 2009, 55, 99–106.
[CrossRef]
21. Buivydaite˙ , V. Classification of Soils of Lithuania Based on FAO-UNESCO Soil Classification System and WRB. In Proceedings
of the 17th World Congress of Soil Science, Bangkok, Thailand, 14–20 August 2002; The Soil and Fertilizer Society of Thailand:
Bangkok, Thailand, 2002; pp. 814–826.
22. Kuliešis, A.; Kulbokas, G.; Kasperavicˇius, A.; Kazanavicˇiu¯ te˙ , V.; Kvalkauskiene˙ , M. Lietuvos nacionaline˙ mišku˛ inventorizacija,
1998–2017. Nuo matavimu˛ iki sprendimu˛. Lithuanian national forest inventory, 1998–2017. In From Measurements to Decision
Making; Lutute˙ : Kaunas, Lietuva, 2021; ISBN 978-9955-37-234-9.
23. Kulbokas, G. Experiences from Lithuania-UNECE Technical Workshop on European Forest Types. In Presented at the UNECE
Technical Workshop on European Forest Types: Reporting Using the New European Forest Types, Bordeaux, France, 19–21 May 2010;
Lithuanian State Forest Service: Kaunas, Lithuania, 2010; pp. 1–16.
24. Angelstam, P. Landscape Analysis as a Tool for the Scientific Management of Biodiversity. Ecol. Bull. 1997, 46, 140–170.
25. Fomin, V.; Mikhailovich, A.; Zalesov, S.; Popov, A.; Terekhov, G. Development of Ideas within the Framework of the Genetic
Approach to the Classification of Forest Types. Balt. For. 2020, 27, 26–39. [CrossRef]
26. Christensen, N.L.; Peet, R.K. Secondary Forest Succession on the North Carolina Piedmont. In Forest Succession: Concepts and
Application; West, D.C., Shugart, H.H., Botkin, D.B., Eds.; Springer Advanced Texts in Life Sciences; Springer: New York, NY,
USA, 1981; pp. 230–245. ISBN 978-1-4612-5950-3.
27. Angelstam, P.; Kuuluvainen, T. Boreal Forest Disturbance Regimes, Successional Dynamics and Landscape Structures: A European
Perspective. Ecol. Bull. 2004, 51, 117–136. [CrossRef]
28. Angelstam, P.; Dönz-Breuss, M. Measuring Forest Biodiversity at the Stand Scale: An Evaluation of Indicators in European
Forest History Gradients. Ecol. Bull. 2004, 51, 305–332. Available online: https://www.jstor.org/stable/20113319 (accessed on
19 September 2022).
29. Jandl, R.; Spathelf, P.; Bolte, A.; Prescott, C.E. Forest Adaptation to Climate Change—Is Non-Management an Option? Ann. For.
Sci. 2019, 76, 48. [CrossRef]
30. Russell, G.J. Turnover Dynamics Across Ecological and Geological Scales. In Turnover Dynamics Across Ecological and Geological
Scales; Columbia University Press: New York, NY, USA, 2001; pp. 377–404. ISBN 978-0-231-50580-2.
31. Neophytou, C.; Heer, K.; Milesi, P.; Peter, M.; Pyhäjärvi, T.; Westergren, M.; Rellstab, C.; Gugerli, F. Genomics and Adaptation in
Forest Ecosystems. Tree Genet. Genomes 2022, 18, 12. [CrossRef]
32. Petrokas, R.; Baliuckas, V.; Manton, M. Successional Categorization of European Hemi-Boreal Forest Tree Species. Plants 2020, 9, 1381.
[CrossRef]
33. Yamamoto, S. Gap Regeneration of Major Tree Species in Different Forest Types of Japan. Vegetatio 1996, 127, 203–213. [CrossRef]
34. Ulft, L. Regeneration in Natural and Logged Tropical Rain Forest-Modelling Seed Dispersal and Regeneration of Tropical Trees in Guyana;
Tropenbos-Guyana Series 12; Tropenbos International: Georgetown, Guyana, 2004; ISBN 90-5113-076-7.
35. Franklin, J. Regeneration and Growth of Pioneer and Shade-tolerant Rain Forest Trees in Tonga. New Zealand J. Bot. 2003,
41, 669–684. [CrossRef]

Diversity 2022, 14, 892
10 of 11
36. Ning, Z.; Hong, J.; Yong-Yan, J. A Phenology Study on the Common Tree Species of Natural Secondary Forests in Northeast
China. Chin. J. Plant Ecol. 1990, 14, 336.
37. Clark, D.A.; Clark, D.B. Life History Diversity of Canopy and Emergent Trees in a Neotropical Rain Forest. Ecol. Monogr. 1992,
62, 315–344. [CrossRef]
38. Whitmore, T.C. Canopy Gaps and the Two Major Groups of Forest Trees. Ecology 1989, 70, 536–538. [CrossRef]
39. Grime, J.P. Evidence for the Existence of Three Primary Strategies in Plants and Its Relevance to Ecological and Evolutionary
Theory. Am. Nat. 1977, 111, 1169–1194. [CrossRef]
40. Hill, M.O.; Roy, D.B.; Thompson, K. Hemeroby, Urbanity and Ruderality: Bioindicators of Disturbance and Human Impact.
J. Appl. Ecol. 2002, 39, 708–720. [CrossRef]
41. Shorohova, E.; Kuuluvainen, T.; Kangur, A.; Jõgiste, K. Natural Stand Structures, Disturbance Regimes and Successional Dynamics
in the Eurasian Boreal Forests: A Review with Special Reference to Russian Studies. Ann. For. Sci. 2009, 66, 201. [CrossRef]
42. Angelstam, P.K. Maintaining and Restoring Biodiversity in European Boreal Forests by Developing Natural Disturbance Regimes.
J. Veg. Sci. 1998, 9, 593–602. [CrossRef]
43. Rull, V. Quaternary Palaeoecology and Ecological Theory. Orsis 1990, 5, 91–111.
44. Hunter, M.L.; Schmiegelow, F.K.A. Wildlife, Forests, and Forestry: Principles of Managing Forests for Biological Diversity, 2nd ed.;
Prentice Hall: Boston, MA, USA, 2011; ISBN 978-0-13-501432-5.
45. Birks, H. Late-Quaternary Biotic Changes in Terrestrial and Lacustrine Environments, with Particular Reference to North-West
Europe. In Handbook of Holocene Palaeoecology and Palaeohydrology; Berglund, B.E., Ed.; Wiley-Interscience; John Wiley & Sons Ltd.:
Chichester, UK, 1986.
46. Birks, H. Contributions of Quaternary Botany to Modern Ecology and Biogeography. Plant Ecol. Divers. 2019, 12, 189–385.
[CrossRef]
47. Godvod, K.; Brazaitis, G.; Bacˇkaitis, J.; Kulbokas, G. The Development and Growth of Larch Stands in Lithuania. J. For. Sci. 2018,
64, 199–206. [CrossRef]
48. Petrokas, R.; Kavaliauskas, D. Concept for Genetic Monitoring of Hemiboreal Tree Dynamics in Lithuania. Land 2022, 11, 1249.
[CrossRef]
49. Odum, E.P.; Barrett, G.W. Fundamentals of Ecology, 5th ed.; Thomson Brooks/Cole: Belmont, CA, USA, 2005; ISBN 978-0-534-42066-6.
50. Navasaitis, M.; Ozolincˇius, R.; Smaliukas, D.; Balevicˇiene˙ , J.M. Lietuvos Dendroflora: Monografija; Lutute: Kaunas, Lietuva, 2003;
ISBN 978-9955-575-35-1.
51. Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al.
Forest Disturbances under Climate Change. Nat. Clim. Chang. 2017, 7, 395–402. [CrossRef] [PubMed]
52. Cochard, H. The Fate of Forest Ecosystems in the Anthropocene. Sigmoidal J. 2020, e008; Preprint. [CrossRef]
53. Shono, K.; Chazdon, R.; Bodin, B.; Wilson, S.; Durst, P. Assisted Natural Regeneration: Harnessing Nature for Restoration.
Unasylva 2020, 252, 71–81.
54. Chai, Y.; Yue, M.; Wang, M.; Xu, J.; Liu, X.; Zhang, R.; Wan, P. Plant Functional Traits Suggest a Change in Novel Ecological
Strategies for Dominant Species in the Stages of Forest Succession. Oecologia 2016, 180, 771–783. [CrossRef]
55. Yu, R.; Huang, J.; Xu, Y.; Ding, Y.; Zang, R. Plant Functional Niches in Forests Across Four Climatic Zones: Exploring the Periodic
Table of Niches Based on Plant Functional Traits. Front. Plant Sci. 2020, 11, 841. [CrossRef]
56. Ehlers, A.; Worm, B.; Reusch, T.B.H. Importance of Genetic Diversity in Eelgrass Zostera Marina for Its Resilience to Global
Warming. Mar. Ecol. Prog. Ser. 2008, 355, 1–7. [CrossRef]
57. Wright, D.; Bishop, J.M.; Matthee, C.A.; von der Heyden, S. Genetic Isolation by Distance Reveals Restricted Dispersal across
a Range of Life Histories: Implications for Biodiversity Conservation Planning across Highly Variable Marine Environments.
Divers. Distrib. 2015, 21, 698–710. [CrossRef]
58. Orr, H.A. Fitness and Its Role in Evolutionary Genetics. Nat. Rev. Genet. 2009, 10, 531–539. [CrossRef]
59. Chazdon, R.; Calixto, B.; Oliveira, M.; Messinger, J.; Alves, J.; Calmon, M.; Anderson, W. The Benefits and Power of Assisted
Natural Regeneration. Available online: https://www.wri.org/insights/what-assisted-natural-regeneration-benefits-definition
(accessed on 19 September 2022).
60. Yang, Y.; Wang, L.; Yang, Z.; Xu, C.; Xie, J.; Chen, G.; Lin, C.; Guo, J.; Liu, X.; Xiong, D.; et al. Large Ecosystem Service Benefits of
Assisted Natural Regeneration. J. Geophys. Res. Biogeosciences 2018, 123, 676–687. [CrossRef]
61. Kpolita, A.; Dubiez, E.; Yongo, O.; Peltier, R. First Evaluation of the Use of Assisted Natural Regeneration by Central African
Farmers to Restore Their Landscapes. Trees For. People 2022, 7, 100165. [CrossRef]
62. A European Commission Initiative Providing Environmental Research Findings in an Easy-to-Understand Way. Available online:
https://environment.ec.europa.eu/research-and-innovation/science-environment-policy_en (accessed on 19 September 2022).
63. Ibanga, D.-A. Is Deep Ecology Inapplicable in African Context: A Conversation with Fainos Mangena. Filos. Theor. J. Afr. Philos.
Cult. Relig. 2017, 6, 101–119. [CrossRef]
64. Akamani, K. Integrating Deep Ecology and Adaptive Governance for Sustainable Development: Implications for Protected Areas
Management. Sustainability 2020, 12, 5757. [CrossRef]
65. Sumarmi, S.; Bachri, S.; Mutia, T.; Yustesia, A.; Fathoni, M.N.; Muthi, M.A.; Nuraini, S.G. The Deep Ecology Perspective of
Awig-Awig: Local Tribal Forest Preservation Laws in Tenganan Cultural Village, Indonesia. J. Sustain. Sci. Manag. 2020,
15, 102–113. [CrossRef]

Diversity 2022, 14, 892
11 of 11
66. Roser, D.; Seidel, C. Climate Justice: An Introduction; Routledge: London, UK, 2016; ISBN 978-1-315-61796-1.
67. Ezemonye, L.I.; Ogbe, M.G. Environmental Issues and Implications of Biofuels as Alternative Sources of Energy. In Critical Issues
on Nigeria’s Development: Environment, Economy and Social Justice; Okafor, F.C., Ed.; Spectrum Books Ltd.: Ibadan, Nigeria, 2011;
pp. 347–376.
