
International Journal of
Environmental Research
and Public Health
Review
Nature-Based Interventions for Psychological Wellbeing in
Long-Term Conditions: A Systematic Review
Eleanor M. Taylor 1,* , Noelle Robertson 1 , Courtney J. Lightfoot 2 , Alice C. Smith 2 and Ceri R. Jones 1,*
1 Department of Neuroscience, Psychology and Behaviour, University of Leicester, Leicester LE1 7HA, UK;
nr6@leicester.ac.uk
2 Leicester Kidney Lifestyle Team, Department of Health Sciences, University of Leicester,
Leicester LE1 7RH, UK; courtney.lightfoot@leicester.ac.uk (C.J.L.); alice.smith@leicester.ac.uk (A.C.S.)
* Correspondence: et200@leicester.ac.uk (E.M.T.); crj10@leicester.ac.uk (C.R.J.)
Citation: Taylor, E.M.; Robertson, N.;
Lightfoot, C.J.; Smith, A.C.; Jones,
C.R. Nature-Based Interventions for
Psychological Wellbeing in
Long-Term Conditions: A Systematic
Review. Int. J. Environ. Res. Public
Health 2022, 19, 3214. https://
doi.org/10.3390/ijerph19063214
Academic Editors: Anna
Maria Palsdottir, Dorthe
Varning Poulsen, Ann Dolling and
Sin-Ae Park
Abstract: Background: With the global burden of disease increasing, particularly in relation to often
preventable chronic diseases, researchers and clinicians are keen to identify interventions that can
mitigate ill health and enhance the psychological wellbeing of people living with long-term conditions
(LTCs). It is long established that engagement with nature can support human health and wellbeing,
and in recent years, nature-based interventions (NBIs) have been advanced as of potential benefit.
This review thus sought to systematically appraise published evidence of the application of NBIs to
address psychological wellbeing for those living with LTCs. Methods: A systematic search of three
databases, PsycINFO, MEDLINE and SCOPUS, was undertaken, and the BestBETs quality assessment
checklist was used to appraise methodological quality of elicited studies. Results: Of 913 studies
identified, 13 studies (12 using quantitative methods, one qualitative) were used. Included papers
reported use of a variety of psychological outcomes alongside more circumscribed physiological
outcomes. Quality appraisal showed modest robustness, some methodological weaknesses and a
dominance of application in developed countries, yet synthesis of studies suggested that reported
psychological and physiological outcomes present a strong argument for NBIs having a promising
and positive impact on psychological wellbeing. Conclusions: NBIs have positive psychological and
physiological impacts on people with LTCs, suggesting they may be a suitable addition to current
maintenance treatment. Future research should focus on minimising study bias and increasing the
potential for cross-cultural applications.
Keywords: long-term conditions; nature-based intervention; systematic review; nature; physical
health
Received: 23 January 2022
Accepted: 3 March 2022
Published: 9 March 2022
Publisher’s Note: MDPI stays neutral
with regard to jurisdictional claims in
published maps and institutional affil-
iations.
Copyright: © 2022 by the authors.
Licensee MDPI, Basel, Switzerland.
This article is an open access article
distributed under the terms and
conditions of the Creative Commons
Attribution (CC BY) license (https://
creativecommons.org/licenses/by/
4.0/).
1. Introduction
Long-term conditions (LTCs) are understood as diseases with no current cure, yet
they require management with drugs or other treatments [1]. Within the UK, those LTCs
most associated with premature death comprise diagnoses of cancer, cardiovascular disease
(CVD), stroke, lung disease and liver disease [2]. Type II diabetes mellitus (T2DM) also
affects 90% of the 3.9 million people currently diagnosed with diabetes in the UK [3] and
is linked to the risk for developing other LTCs [2]. Indeed, uncontrolled diabetes and
high blood pressure are the biggest known causes of chronic kidney disease (CKD) [4],
which affects approximately three million people in the UK. Lifestyle factors, such as
poor diet, physical inactivity, drinking alcohol and smoking, can impact disease morbidity
and progression [2]. These behaviours increase the risk of developing many LTCs and
are modifiable.
Given the enduring and costly nature of LTCs, with in excess of 15 million people in
England living with LTCs [1,2], there is a need to improve treatment options. For example,
data from the last decade suggest that LTCs account for 50% of GP appointments, 64%
of outpatient appointments and 70% of inpatient bed days in England alone [5]. LTCs
Int. J. Environ. Res. Public Health 2022, 19, 3214. https://doi.org/10.3390/ijerph19063214
https://www.mdpi.com/journal/ijerph

Int. J. Environ. Res. Public Health 2022, 19, 3214
2 of 23
also affect individuals disproportionately; for example, 58% of those living with LTCs are
over 60 years old, while the chance of developing an LTC is 60% more likely and will be
30% more severe for those in the lowest socio-economic groups compared to those in the
highest [5].
In an increasingly urbanised world, with a trend for population movement to cities,
there is a suggestion that engagement with and exposure to nature is beneficial. For
example, those living in areas close to greenspace have a reduced mortality risk [6–8].
Greenspace proximity is also associated with a reduced incidence of neurological disor-
ders [9], CVD [7] and T2DM [10]. A recent review of systematic reviews of the relationship
between public health and proximity to nature concluded that being closer to greenspace
reduces the incidence of stroke, hypertension, dyslipidaemia, asthma and coronary heart
disease [11]. Even bringing nature inside has shown advantages for health and wellbeing;
for example, Ulrich’s seminal study [12] demonstrated the benefit of views of nature for
post-surgical recovery.
The advantageous effects of natural world experiences on human health and wellbeing
are increasingly well-established [13]; however, the mechanisms affecting these improve-
ments remain speculative [14]. A dominant putative mechanism is advanced through
the biophilia hypothesis, which suggests that humans are innately attracted to natural
environments [15], and evolutionary psychology proposes that the human brain and body
have been shaped by millions of years living in nature [16]. This intrinsic appeal has fuelled
interest in and the development of nature-based interventions (NBIs) to improve health
and wellbeing.
NBIs are a diverse array of activities or programmes aimed at engaging individuals
in nature-based experiences to improve health and wellbeing. Common NBIs include
horticultural therapy, which has been shown to improve general wellbeing [17], as well as
mood and performance for people with mental health conditions [18]. Forest therapy is also
understood as a method of NBI, most notably in the form of “forest bathing” or Shinrin Yoku
(reflecting its origins). This involves experiencing the calm and quiet of trees for relaxation
with significant benefits for both physical and mental health [19] and human immune
function [20], an effect not replicated in an urban comparison [21], as well as improved
self-efficacy, life satisfaction and physical activity and reduced unhealthy eating in young
people [22]. The beneficial effects of NBIs appear not just attributable to increased physical
activity since even short-term visits to urban greenspaces can reduce blood pressure and
heart rate variability in comparison to visiting urban streets [23]. In addition to these
physiological benefits, there are also psychological benefits of interaction with nature. For
example, cognition can also be enhanced, with studies reporting improved Stroop test
performance from walking in natural as opposed to built-up environments [24], as well as
improved cognitive performance in individuals with major depressive disorder [25].
Incorporated within NBIs are also those which feature human–animal interactions in
outdoor environments, involving specially trained animals and therapeutic goals. Previous
reviews have revealed the positive effect of animal-assisted interventions for physical and
psychological wellbeing [26–29], as well as the ability to cope with stress, improvements
to cardiovascular health, and maintaining health and mobility in older age [30]. More
specifically, in addition to mechanical benefits [31,32] such as improved motor ability,
independence of ambulation and gait [33], enhanced quality of life (QoL) has been reported
following horse therapy for stroke survivors [34]. Horse therapy also appears to confer
psychological benefits for those living with chronic back pain who report increases in
positive affect and meaningful activities [35], as well as facilitating positive self-identity in
people with physical disabilities [36].
Given that a preliminary scoping review revealed no previous overarching review
of published evidence examining how NBIs have been offered for those living with LTCs,
our review seeks to systematically appraise, synthesise and evaluate published research
examining the impact of NBIs on psychological wellbeing. This hopes to identify the extent
of work to date which has deployed NBIs and with what effects, assess nature’s impact

Int. J. Environ. Res. Public Health 2022, 19, 3214
3 of 23
on wellbeing and its potential to benefit self-management and contribute to the menu of
interventions addressing health outcomes, particularly of psychological wellbeing.
2. Materials and Methods
2.1. Study Selection
This review focused on the most prevalent LTCs associated with premature death [2]
but excluded cancers (thus CVD, stroke, lung disease and liver disease). The review
also included CKD and T2DM, given their population prevalence. Cancer studies were
excluded from the present review because initial scoping identified sufficient number of
papers to warrant a separate review. Additionally, given cancers are not exclusively caused
by lifestyle factors, nor are they always incurable, they are arguably outside of the focus of
this review. This review considered NBIs if the interventions contained a nature or green
element, whether conducted inside or outside. Animal-based interventions conducted
inside, for example, pet-assisted therapy conducted at a hospital bedside, were not included
in this review as they did not meet the inclusion criteria of containing a nature or green
element. However, those delivered within an outdoor natural environment were included.
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
• Nature-based intervention conducted inside or outside or animal-based intervention
conducted in outdoors environments;
• Involves an active intervention rather than just passive proximity to, e.g., greenspace;
• Studies with a minimum of 50% participants with at least one diagnosed long-term
physical health condition, limited to cardiovascular disease, stroke, lung and liver dis-
ease, type II diabetes or chronic kidney disease, but not excluding other co-morbidities;
• Measuring psychometrically robust psychological outcomes, including (but not limited
to) QoL.
2.2.2. Exclusion Criteria
• Not specific to long-term conditions listed above;
• Participants within end-of-life pathways (e.g., palliative care).
2.3. Search Strategy
The search strategy was based on the PICO framework [37] (Table 1). A title, abstract
and keyword search (see Table 2 for terms) was performed on the following electronic
databases in January 2022: PsycINFO, MEDLINE and Scopus. Search limitations included
English language and human participants, but the search was not limited to peer-reviewed
publications only. After duplicate removal, a manual search of the titles and abstracts was
performed using the eligibility criteria listed above.
Table 1. PICO framework.
P—Population
I—Intervention
C—Comparison
O—Outcome
PICO
People with long-term physical health conditions limited to cardiovascular
disease, stroke, lung and liver disease, type II diabetes and chronic
kidney disease
Nature-, green- or outdoor animal-based interventions
Treatment as usual, urban environments or no comparison
Evaluation of effectiveness, as measured by improvements in psychological
wellbeing and/or quality of life (QoL)

Int. J. Environ. Res. Public Health 2022, 19, 3214
4 of 23
Table 2. Free text search terms for nature- and animal-based interventions with long-term conditions.
These were combined with the Boolean operator AND.
Search Terms
Nature-based interventions
((garden* OR green OR horticultur* OR “nature-based” OR
“nature based”) N2 (therap* OR intervention* OR proximity)) OR
((healing OR restorative OR wander) N2 garden) OR “green
prescri*” OR “social prescri*” OR “nature prescri*” OR “nature
play” OR “park prescri*” OR “garden prescri*” OR “green space*”
OR greenspace* OR “green exercise” OR “green infrastructure”
OR “community garden*” OR “community allotment*” OR
allotment* OR “outdoor exercise” OR “blue space*” OR “blue
gym*” OR “green gym*” OR “park prescri*” OR “eco therapy”
OR “eco-therapy” OR “wilderness therapy” OR
“wilderness-therapy” OR “care-farming” OR “care farming” OR
“farm therapy” OR “farm-therapy” OR “forest bathing” OR
“forest-bathing” OR “environmental volunteering” OR “wild
play” OR “nature play” OR “animal assisted therap*” or
“animal-assisted therap*” OR “animal therap*” OR “pet therap*”
or “pet-assisted therapy” OR “equine assisted therap*” OR
“equine-assisted therap*” OR “canine assisted therap*” OR
“canine-assisted therap*”
Long-term conditions
Cardiovascular or “cardiovascular disease” or Hypertension or
“high blood pressure” or “Coronary Heart Disease” or “heart
disease” or CHD or “Coronary Disease” or “vascular disease” or
“Heart failure” or “Pulmonary Heart Disease” OR “Pulmonary
disease” or “Respiratory disease” or Asthma or “Chronic
Obstructive Pulmonary Disease” or COPD OR “Liver disease” or
“Chronic liver disease” or “liver cirrhosis” or “Fatty Liver” or
Hepatitis or “hepatic disease” OR “type II diabetes” or “type two
diabetes” or “type 2 diabetes” or Diabetes or T2DM or “diabetes
mellitus” OR “Kidney disease” or “Chronic kidney disease” or
CKD or “renal insufficiency” or “chronic renal insufficiency” or
“renal disease” or “chronic renal disease” or “kidney failure” or
“renal failure” or AKI or “acute kidney injury”
* Truncation symbol which when used at the end of search terms finds any string of characters in that position; for
example, therap* would identify therapist, therapies, therapy etc.
2.4. Data Extraction
Shortlisted articles were read in full by the first author, and a data extraction table
adapted from Brooks et al. [38] was used to synthesise data relevant to this review (see
Table 3 for a summary). The 12 full-text articles that were excluded prior to analysis were
deemed not appropriate for inclusion. Reasons for exclusions included: not having a
condition that matched the inclusion criteria for an LTC (n = 5), not containing a nature or
green element (n = 4), protocol-only papers and did not contain any data (n = 2) or deemed
poor quality as it did not report any data (n = 1).
Variables reported were publication year, study design, sample size and participant
demographics, type of LTC, type of NBI, psychological and physiological measures used
and their outcomes, and country of study. Key information on each study’s main aims were
also extracted where they were relevant to the review question. Papers were then grouped
by the LTC of participants. Eleven out of thirteen studies included in this review also
investigated physiological measures in addition to psychological measures. These are also
included in the analysis because understanding is still evolving regarding the bidirectional
impacts of psychological and physiological processes.
2.5. Quality Appraisal
The BestBETs quality assessment checklist [39], chosen from the Systematic Review
Toolbox [40] as recommended by Booth et al. [41], was used to assess the methodological
also investigated physiological measures in addition to psychological measures. These are
also included in the analysis because understanding is still evolving regarding the bidi-
rectional impacts of psychological and physiological processes.
Int. J. Environ. Res2..P5u. bQlicuHaleiatlythA20p2p2r, a1i9s,a3l214
The BestBETs quality assessment checklist [39], chosen from the Systematic Review
5 of 23
Toolbox [40] as recommended by Booth et al. [41], was used to assess the methodological
quality of the 12quqaulaitnytiotafttihvee 1s2tuqduieasnteiltiactiitvede.sTtuhdisietsoeollicpirteodv.idTehsicsrtiotiocal lparopvpirdaeissaclrfiotircaal appraisal for a
range of study tyrapnegs,ewohf iscthudwyatsyspueist,ewd htoicthhewdaisvseursiteedstutodythdeedsiivgenrssienstthuedyprdeesseingtnrseivnietwhe present review
and is designedafnodr cios hdoerstighneeadlthfocracroehroesrteahrecahlt.hIctacroemrepsreiasercsh3.3Ititceommspervisaelsu3a3tinitgemdsomevaailnusating domains of
of objectives andobhjeycptoivthesesaensd, dheyspigonth, emseesa,sduerseimgne,nmt aenadsuorebmseernvtatainodn,opbrseesrevnattaiotino,nporefsreen-tation of results,
sults, analysis, daisncaulysssiios,nd, iisnctuesrpsiroenta, itniotnerapnredtaimtiopnleamndenitmatpiolenm. Fenotraetaiocnh.iFteomr e, astcuhdiiteesmw, setruedies were scored
scored 0 (not re0po(nrtoetdr)e,p1or(rteedpo),r1te(drebpuotrtiendadbeuqtuinaated)eoqrua2te(r)eopro2rt(erdepaodreteqduaatdeelyq)u, awteitlhy),awith a potential
potential total sctootrael socfo6r4e o(sfe6e4 S(suepepSluepmpelnemtaeryntTaraybTleabSl1e).S1T)h. eThoeneonqeuqauliatalittiavteivsetustduydywwasas assessed using
assessed using aagguuidideefoforrrreeaaddininggqquuaaliltiatatitviveesstutuddieiesspprreesseennteteddbbyySSaannddeelolowwsskkiiaannddBBaarr-roso [42], which
roso [42], whicheennccoouurraaggeessrreeaaddeerrssooffqquuaalliittaattiivveerreesseeaarrcchhttoottaakkeemmeeaanniinnggffrroommtthheetteexxttover employing
over employingssttrriiccttssttaannddaarrddssaannddccrriitteerriiaa..
3. Results
3. Results
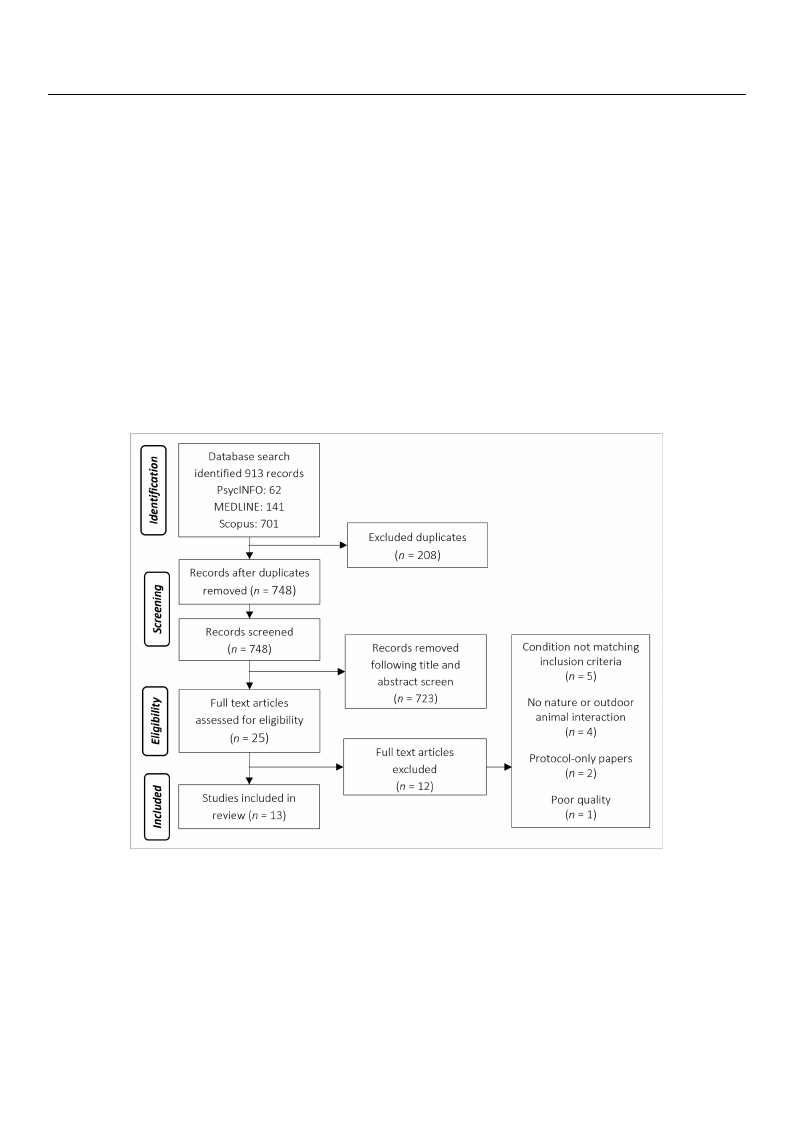
The database seTarhcehdiadteanbtaifsiedse9a1r3chstiuddenietsi.fiFedoll9o1w3 isntguddiuesp.liFcoaltleorweimngovdaul,palicmaatenureaml oval, a manual
search of title ansdeaarbcshtroafctistliedeantdifaiebdst2r5acsttsuiddiens,tiwfiehdic2h5restcuedivieds, awfhuilclh-praepcerivredviaewfu.lTl-hpeaper review. The
search also identsiefaierdchaaslyssoteidmeanttiicfireedviaeswysotfesmyastiecmreavtiicerwevoifeswysst[e4m3]a, twichriecvhiewwas [m43a]n,uwahlliych was manually
searched and idseenatrifciheeddaanfudrtihdernttiwfieodsatufduiretsh.eIrntwtootaslt,u1d3iesst.uIdnietostwal,er1e3 estliugdibielse wfoerreineclliugi-ble for inclusion:
sion: twelve quatnwtietlavteivqeuaanndtitoanteivqeuaanlditaotniveeq(useaelitPaRtiIvSeM(sAeeflPoRwISdMiaAgraflmow, Fdigiaugrrea1m; ,[4F4ig])u. re 1; [44]).
Figure 1. PRISMAFfilgouwred1ia. gPrRaImSMdAepflicotiwngdtiahgerparmocdeesps iocftisncgretehneinpgro. cess of screening.
3.1. Study Characteristics
The 13 studies identified by this review were published between 2005 and 2020 and
reported on a total of 512 participants (see Table 3 for a summary of study characteristics).
Eight studies recruited both males and females [34,45–51], four recruited only males [52–55]
and one did not specify sex [56]. Ten studies were conducted in Asia (including Korea,
China or Japan), and one each within the USA, Sweden and Brazil. Most of the studies
did not specify how they recruited their participants, although the majority appeared
to use opportunistic sampling, while three explicitly stated advertisement using either
posters [45], newspapers [53] or within local health centres [48,54].

Int. J. Environ. Res. Public Health 2022, 19, 3214
6 of 23
Table 3. Summary of study characteristics.
Author
(Year)
Research
Design
Beinotti et al.
(2013)
[34]
Single-blind
RCT
Chun et al.
(2017)
[45]
Two-sample
randomised
cohort
Pohl et al.
(2018)
[46]
Qualitative
exploration
Jia et al.
(2016)
[47]
Two-sample
randomised
cohort
Participants
Intervention
20 patients
(6 female)
≥1 year
post-stroke
Mean age
55.5 years
16 weeks physio
and horse-riding
therapy
(10 participants)
Mean age 59 years
59 participants
(19 female)
Mean age
60.8 years
(SD 9.1)
Forest therapy
programme
(30 participants)
4-day trip involving
meditation,
experiencing the
forest and walking
18 participants
(6 female)
Mean age 60.3
1–5 years
post-stroke
12-week
multi-modal
intervention
incorporating
horseback riding
20 COPD
patients
(6 female)
Mean age
70.1 years
Forest walking
(10 participants)
Mean age 70.1
(range 67–77)
7 days at forest site
with scheduled
walking time,
staying in a hotel
Control
16 weeks physio
only
(10 participants)
Mean age
52 years
Urban
comparison
(29 participants)
4-day trip
involving
meditation and
walking in the
hotel
No comparison
group
City walking
(8 participants)
Mean age 70
(range 61–79)
7 days at city site
with scheduled
walking time,
staying in a hotel
Psychological
Measurements
Medical Outcomes Study
36-item Short-Form
health survey
Measurements before
and after the intervention
Beck Depression
Inventory (BDI)
Hamilton Depression
Rating Scale (HAM-D17)
Spielberger State-Trait
Anxiety Inventory (STAI)
Measurements before
and after the intervention
Individual face-to-face
semi-structured
interviews
Profile of Mood
State (POMS)
Measurements before
and after the intervention
Psychological
Outcomes
Significant improvement
in functional capacity,
physical aspects and
mental health following
horseback riding therapy
compared to controls.
No changes in general
health state, vitality or
emotional aspects
Reduced BDI,
HAM-D17 and STAI
scores following forest
therapy programme
compared to baseline.
Increased STAI scores in
urban group
following programme
Four themes identified:
transformative
experiences,
human–horse
interaction,
togetherness and
belonging, and the
all-in-one solution
Lower POMS scores of
“tension–anxiety”,
“depression” and
“anger–hostility” in
forest but not city group
Physiological
Measurements
N/A
Oxidative stress: total oxidant
capacity and
iron-reducing activity
Measurements before and after
the intervention
N/A
Lymphocytes: NK, NKT-like and
CD8+ T-cells expression of
intracellular perforin and
granzyme B Pro-inflammatory
cytokines: interferon-γ (IFN-γ),
interleukin-6 (IL-6), interleukin-8
(IL-8), interleukin-1β (IL-1β),
tumour necrosis factorα
(TNF-α) and C-reactive protein
(CRP) COPD-associated factors:
pulmonary and
activation-regulated chemokine
(PARC/CCL-18), surfactant
protein D (SP-D) and tissue
inhibitor of metalloproteinase
(TIMP-1) Stress hormones:
serum cortisol and epinephrine
Measurements before and after
the intervention
Physiological Outcomes
N/A
No significant differences between
forest and urban groups
N/A
Lymphocytes: no significant group
difference in proportion of NK,
NKT-like and CD8+ T-cells, nor their
expression of granzyme B. Significant
reduction of NK, NKT-like and CD8+
T-cell expression of intracellular
perforin after forest bathing but not city
group. Pro-inflammatory cytokines:
significant reduction of IFN-γ, IL-6 and
IL-8 after forest bathing but not city
group. Slight decrease in IL-1β, TNF-α
and CRP after forest bathing but not
city group. COPD-associated factors:
significant decrease in PARC/CCL-18
TIMP-1 after forest bathing but not city
group. No significant change in SP-D in
either group. Stress hormones:
significant decrease in serum cortisol
and epinephrine after forest bathing
but not city group
Int. J. Environ. Res. Public Health 2022, 19, 3214
7 of 23
Author
(Year)
Research
Design
Table 3. Cont.
Participants
Intervention
Song et al.
(2015)
[52]
Two-sample
randomised
cross-over
cohort study
20 male
participants
with
high-normal
blood
pressure
(HNBP) or
hypertension
Mean age 58.0
years
(SD 10.6)
Forest walking
17 min walk
All participants
completed both
interventions on
2 consecutive days
(10 in each group,
counterbalanced:
forest first vs
urban first)
Li et al.
(2016)
[53]
Single-
sample
cross-over
cohort study
19 male
participants
with
high-normal
blood
pressure
(HNBP) or
hypertension
Mean age
51.2 years
(SD 8.8)
Forest walking
Day trip
All participants
completed both
interventions.
Urban first
Control
Urban walking
17 min walk
Urban walking
Day trip
Psychological
Measurements
Semantic Differential
(SD) method Profile of
Mood State (POMS)
Measurements taken at
end of each walk
Profile of Mood
State (POMS)
Measurements taken
before, during and after
each intervention
Psychological
Outcomes
Increased SD scores of
“comfortable”, “relaxed”
and “natural” after
waking in forest area
compared with urban
area. Reduced negative
POMS scores of
“tension–anxiety”,
“depression”,
“anger–hostility”,
“fatigue” and
“confusion”, with
increased “vigour” after
walking in forest area
compared to urban area
Reduced POMS (D), (A),
(F), (C) and increased
(V) in forest walking but
not city walking. City
group also had
increased (D)
Physiological
Measurements
Heart rate variability (HRV) and
heart rate
Measures collected at 1 min
intervals and averaged over the
17 min course
Blood pressure and heart rate.
Serum triglycerides, total
cholesterol (Cho), low-density
lipoprotein (LDL) Cho,
high-density lipoprotein (HDL)
Cho and remnant-like particles
(RLP) Cho, serum adiponectin,
blood glucose, serum insulin,
serum dehydroepiandrosterone
sulphate (DHEA-S), serum
high-sensitivity C-reactive
protein (hs-CRP). Urinary
adrenaline, noradrenaline and
dopamine (corrected
for creatinine)
Blood and urine collected in the
morning before and after each
day trip. Blood pressure and
heart rate measured by an
ambulatory monitor every
20 min
Physiological Outcomes
Significantly higher parasympathetic
activity during forest walking
compared to urban walking. No
significant difference in sympathetic
nerve activity between groups.
Significantly lower mean heart rate
during forest walking compared to
urban walking. Physiological measures
were significantly related to the
differences in air temperature and
humidity between the forest and
urban environments
No significant difference in blood
pressure between forest and urban
day trips.
Significant decrease in heart rate
during forest walking compared to
urban walking. No significant change
in serum triglycerides, Cho, LDL Cho,
HDL Cho, and RLP Cho, blood glucose,
serum insulin, serum DHEA-S, or
hs-CRP. Significant increase in serum
adiponectin after forest but not urban
day trips.
Both forest and urban walking
significantly reduced urinary
noradrenaline.
Non-significant decrease in urinary
adrenaline after forest walking
compared to urban walking.
Significant decrease in urinary
dopamine after forest walking
compared to urban walking

Int. J. Environ. Res. Public Health 2022, 19, 3214
8 of 23
Table 3. Cont.
Author
(Year)
Ochiai
et al. (2015)
[54]
Song et al.
(2017)
[55]
Sung et al.
(2012)
[48]
Wu et al.
(2020)
[49]
Research
Design
Single-
sample
cohort
Two-sample,
randomised
cross-over
cohort
Non-
randomised
controlled
trial
Two-sample
randomised
cohort
Participants
9 male
participants
with
high-normal
blood
pressure
(HNBP) Mean
age 56.0 years
(SD 13.0)
20 males with
high-normal
blood
pressure or
hypertension
Mean age
58.0 years
(SD 10.6)
56 participants
(22 female)
with
hypertension
Mean age
64.5 years
31 participants
(12 female)
with
hypertension
Mean age
73.7 years
Intervention
Forest therapy
1-day programme
involving walking,
sitting and
lying down
Viewing forest
landscape for
10 min while sitting
(10 participants saw
forest first, 10 saw
urban first on
2 consecutive days)
3-day CBT Forest
Therapy
programme
including
2 recreational visits
to forest sites
(28 participants)
Mean age 63 years
(SD 11) 50% male
Forest walking
(20 participants)
2 days at forest site
with scheduled
walking, rest and
staying in a hotel
Control
No comparison
group
Viewing urban
landscape for
10 min
while sitting
Provided with
printed
educational
materials for
hypertension
management
(28 participants)
Mean age 66
years (SD 7)
28% male
City walking
(11 participants)
2 days at city site
with scheduled
walking, rest and
staying in a hotel
Psychological
Measurements
Semantic Differential
(SD) method Profile of
Mood State (POMS)
combined POMS Total
Mood Disturbance
(TDM)
Measurements before
and after the intervention
Psychological
Outcomes
Increased SD scores of
“relaxed” and “natural”
after forest therapy
compared with baseline.
Reduced negative
POMS scores of
“tension–anxiety”,
“confusion” and
“anger–hostility”, and
TDM after
forest therapy.
Modified semantic
differential (SD) method
completed after
each viewing
Significantly increased
scores of “comfortable”,
“relaxed” and “natural”
after viewing forest area
compared to urban area
QoL with 5 domains:
General Health (GH),
Physical Dimension (PD),
Mental Dimension (MD),
Social Dimension
(SD and
Hypertension-related
Dimension (HTD).
Measured at initial visit
and at 8-week final visit
Forest group showed
significantly increased
total QoL scores after
forest therapy. Increases
in MD and HTD but not
GH or SD. No
significant change in
control group
Profile of Mood
State (POMS)
Measurements before
and after the intervention
Reduced negative
POMS scores of
“tension–anxiety”,
“depression”,
“confusion” and
“fatigue”, as well as
increased “vigour” after
forest bathing compared
to city walking group.
Physiological
Measurements
Blood pressure Urinary
adrenaline (corrected for
creatinine) Serum cortisol
Blood pressure collected during
intervention using portable
device. Urine and blood samples
collected in the afternoon before
and after the intervention
Heart rate variability (HRV) and
heart rate collected at 1 min
intervals and averaged across
the 10 min period
Blood pressure: measured at
start and at end of 3-day
program. Daily self-monitoring
morning and evening from first
until last day of study
Salivary cortisol: collected at
initial visit and 8-week final visit
Blood pressure, heart rate,
oxygen saturation (SpO2%) and
heart rate variability (HRV)
Measurements taken in the
morning before and after
the intervention
Physiological Outcomes
Significant decrease in blood pressure
after forest therapy. Significant
decrease in urinary adrenaline and
serum cortisol after forest therapy
Significantly increased high-frequency
HRV during forest compared to urban
viewing. No significant
difference between
high-frequency/low-frequency heart
rate significantly lower during forest
compared to urban viewing
Blood pressure: marginally
significantly larger decrease in systolic
blood pressure following forest therapy
(at day 3). No change in diastolic blood
pressure or either of self-measured at
week 4 or 8. No significant longitudinal
change in blood pressure in
either group
Salivary cortisol: significantly larger
reduction following forest therapy and
significant increase in control group
Significant decrease in diastolic blood
pressure, but not systolic blood
pressure after forest bathing compared
to controls Significant increase in
SpO2% after forest bathing compared
to controls No significant change in
heart rate Significantly decreased in LF
HRV and LF/HF HRV after forest
bathing compared to controls.
Significant increase in HF HRV after
forest bathing compared to controls

Int. J. Environ. Res. Public Health 2022, 19, 3214
9 of 23
Author
(Year)
Research
Design
Table 3. Cont.
Participants
Intervention
Control
Mao et al.
(2012)
[56]
Two-sample
randomised
cohort
24 patients
with essential
hypertension
(does not
specify sex
or age)
Forest walking
(12 participants)
7 days at forest site
with scheduled
walking time,
staying in a hotel
City walking
(12 participants)
7 days at city site
with scheduled
walking time,
staying in a hotel
Mao et al.
(2017)
[50]
Two-sample
randomised
cohort
33 participants
(14 female)
with Chronic
Heart Failure
Mean age
71.8 years
Forest walking 23
participants
4-day trip
City walking
10 participants
4-day trip
Psychological
Measurements
Profile of Mood
State (POMS)
Measurements before
and after the
intervention.
Profile of Mood
State (POMS)
Measurements before
and after the
intervention.
Psychological
Outcomes
Lower POMS scores of
“depression”,
“anger-hostility”,
“fatigue” and
“confusion”, with
increased “vigour” in
forest but not city group
Reduced negative
POMS scores of
“tension–anxiety”,
“depression”,
“anger–hostility” and
“confusion” compared
to baseline in forest
group but not
city group.
Physiological
Measurements
Blood pressure and heart rate
Cytokines: homocysteine (Hcy),
constituents of the
renin-angiotensin system (RAS)
including renin, angiotensinogen
(AGT), angiotensin II (Ang II),
angiotensin II type 1 receptor
(AT1), angiotensin II type 2
receptor (AT2) Cardiovascular
disease-associated factors: serum
interleukin-6 (IL-6), tumour
necrosis factorα (TNF-α) and
endothelin-1 (ET-1)
Measurements taken in the
morning before and after
the intervention
High-sensitive-reactive protein
(hCRP) Bio-markers for heart
failure: BNP and NT-ProBNP
Cardiovascular disease-related
factors: ET-1, constituents of the
renin–angiotensin system (RAS)
including renin, angiotensinogen
(AGT), angiotensin II (Ang II),
angiotensin II type 1 receptor
(AT1), angiotensin II type 2
receptor (AT2) Pro-inflammatory
cytokines: interleukin-6 (IL-6),
tumour necrosis factorα (TNF-α)
Oxidative indicators: activity for
serum total SOD (T-SOD) and
lipid peroxidation reflected by
malondialdehyde (MDA)
Measurements taken in the
morning before and after
the intervention
Physiological Outcomes
Significant decrease in systolic and
diastolic blood pressure after forest
bathing compared to controls No
significant change in heart rate
Significant decrease in ET-1 and Hcy,
RAS constituents including AGT, AT1,
and AT2 after forest bathing compared
to controls. Non-significant reduction
in renin and Ang II after forest bathing
compared to controls. Significant
association between systolic blood
pressure and Ang II, ET-1 and Hcy.
Diastolic blood pressure was
significantly associated with Ang II and
ET-1. BP was poorly associated with
the change in renin, AT1, and AGT
Significant decrease in serum IL-6 after
forest bathing compared to controlsNo
significant change in TNF-α
Significant decrease in BNP after forest
bathing compared to baseline. No
significant difference in controls. No
significant difference in NT-ProBNP in
either group. Significant decrease in
ET-1 after forest bathing compared to
controls. No change in ET-1 and the
five RAS constituents in the city group
compared to baseline. Significant
increase in AT2 after forest bathing
compared to baseline. No significant
difference in controls Significant
decrease in serum IL-6 after forest
bathing compared to controls. No
significant changes in TNF-α or
high-sensitive-reactive protein (hCRP)
Significant decrease in serum MDA and
significant increase in T-SOD after
forest bathing compared to controls.
No significant difference in controls
Int. J. Environ. Res. Public Health 2022, 19, 3214
10 of 23
Table 3. Cont.
Author
(Year)
Research
Design
Participants
Intervention
Wichrowski
et al. (2005)
[51]
Two-sample
cohort non-
randomised
107 participants
(42 females)
Inpatients on
a phase I
cardiac
rehabilitation
programme
(does not
specify age)
Horticultural
therapy
59 participants
single session
Control
Patient education
class
48 participants
single session
Psychological
Measurements
Profile of Mood State
(POMS) combined POMS
Total Mood
Disturbance (TDM)
Measurements before
and after the intervention
Psychological
Outcomes
Reduced negative
POMS scores of
“tension”, “depression”,
“anger”, “confusion”
and “fatigue”, as well as
increased “vigour” after
horticultural therapy.
TMD decreased after
horticultural therapy.
No changes following
patient education class.
Physiological
Measurements
Heart rate before and after
intervention
Physiological Outcomes
Significant decrease in heart rate
following horticultural therapy, but no
significant change following patient
education class

Int. J. Environ. Res. Public Health 2022, 19, 3214
11 of 23
The LTCs reported were chronic obstructive pulmonary disease (COPD; n = 1) [47],
stroke (n = 3) [34,45,46] and CVD (n = 9) [48–56]. The cardiovascular conditions comprised:
high-normal blood pressure (HNBP) or hypertension (n = 7) [48,49,52–56], chronic heart
failure (n = 1) [50], and those included in a cardiopulmonary rehabilitation programme for
post cardiac surgery, post myocardial infarction or congestive heart failure (n = 1) [51].
The NBIs reported consisted of horticultural therapy (n = 1) [51], forest-based inter-
ventions (e.g., forest bathing, forest walking, forest therapy, CBT forest therapy or viewing
a forest landscape; n = 10 [45,47–50,52–56]) and horse-riding therapy (n = 2) [34,46]. Forest-
based interventions, such as forest bathing and forest walking, ranged from a single 17 min
walk to a 7-day trip. Horse-riding interventions were either 12- or 16-week programmes of
weekly sessions, while horticultural therapy consisted of a single 60 min session.
Eleven studies had a control comparison group. Forest-based interventions used an
urban or city comparison [45,47–50,53,55,56] or, in the case of the CBT forest therapy [48],
provided control participants with printed educational material only. Horticultural therapy
was compared to a patient education class [51]. One horse-riding study compared horse
riding and physiotherapy with physiotherapy alone [34]. The studies without a comparison
group were one forest study [54] and the qualitative horse-riding study [46]. However,
the latter interviewed only participants who took part in the horse-riding arm of a wider
randomised control trial (RCT) investigating horse-riding therapy and rhythm and music-
based therapy for stroke survivors in late-phase recovery [57,58].
3.2. Psychological Outcomes
Twelve studies used quantitative measures to assess the psychological impact of the
NBIs. These included the Profile of Mood States (POMS) [59], the Medical Outcomes Study
36-item Short-Form health survey (SF-36) [60], the Semantic Differential (SD) method [61],
the Beck Depression Inventory (BDI) [62], the Hamilton Depression Rating Scale (HAM-
D17) [63], the Spielberger State-Trait Anxiety Inventory (STAI) [64], and a QoL measurement
tool [65] based on the SF-36 and the Duke-UNC Health profile. Other than the SF-36, used
only in Beinotti et al.’s [34] single-blind randomised trial by a researcher–rater blind to
intervention, all were self-reported measures.
The POMS was used in eight studies, all of which reported improvement follow-
ing NBIs, although no domain was consistently improved (see Table 4). Decreases in
POMS negative domains were reported after a single day of forest therapy in HNBP or
hypertension participants [54], as well as when compared to urban or city comparisons
in COPD patients [47], HNBP or hypertension participants [49,52,53,56] and chronic heart
failure patients [50]. Li et al. [53] also found significant increases in depression in their
city comparison group. An increase in the positive domain “vigour” was also observed
when compared to urban or city conditions [49,52,53,56]. Horticultural therapy for car-
diopulmonary rehabilitation inpatients was reported to decrease negative POMS scores
and increase positive “vigour” scores [51]. Additionally, two studies reported significantly
reduced total mood disturbance scores following the intervention [51,54].
Beinotti et al. [34] found a significant improvement in functional capacity, physical
aspects and mental health factors of the SF-36 in stroke survivors following horse-riding
therapy, compared to controls. However, no changes in general health state, vitality or
emotional aspects were observed. Measures of “relaxed” and “natural” were increased
following forest therapy [54], in addition to “comfortable” scores when compared to an
urban condition in participants with HNBP or hypertension [52]. Chun et al. [45] was
the only study to measure depression (BDI and HAM-D17) and anxiety (STAI), reporting
significant reductions in all three measures from baseline following forest therapy in stroke
survivors. One study [51] used a horticultural therapy intervention, while all others used
forest therapy interventions.

Int. J. Environ. Res. Public Health 2022, 19, 3214
12 of 23
Table 4. Summary of POMS positive outcomes after the intervention (decreased negative, increased
positive) by domain. + denotes this domain was significantly improved following the intervention.
Negative
Positive
Author (Date) [Ref]
Jia et al. (2016) [47]
+
+
+
Song et al. (2015) [52]
+
+
+
+
+
+
Li et al. (2016) [53]
+
+
+
+
+
Ochiai et al. (2015) [54]
+
+
+
Wu et al. (2020) [49]
+
+
+
+
+
Mao et al. (2012) [56]
+
+
+
+
+
Mao et al. (2017) [50]
+
+
+
+
Wichrowski et al. (2005) [51]
+
+
+
+
+
+
3.3. Physiological Outcomes
Eleven studies included in this review also assessed the physiological impact of NBIs
in addition to psychological outcomes. Outcomes measures included blood pressure, heart
rate, oxygen saturation, heart rate variability (HRV) and stress hormone secretion, as well
as proportions of lymphocytes and pro-inflammatory cytokines. Factors associated with
risk for developing COPD [47] and CVD [50,53,56], such as blood cholesterol, C-reactive
protein, and NT-ProBNP, were also measured.
Impacts on heart rate (HR) were reported in eight studies. Three forest-based inter-
ventions reported significant decreases in HR [52,53,55], while HR of cardiopulmonary
rehabilitation patients also decreased significantly following horticultural therapy [51].
Conversely, two studies that measured HR before and after forest walking reported no
significant change following the intervention [49,56]. Three studies reported significantly
higher parasympathetic activity during forest walking [49,52,55], and one also reported
reduced sympathetic activity [49].
Blood pressure was also measured in five studies. Findings showed a significant
decrease in blood pressure after forest therapy [54], a significant decrease in diastolic but
not systolic blood pressure after forest bathing [49], and a significant decrease in diastolic
and systolic blood pressure after forest bathing [56]. However, Li et al. [53] found no
significant difference in blood pressure between forest and urban walking conditions,
and Sung et al. [48] reported only a marginally significant decrease in systolic blood
pressure following viewing forest landscapes. Additionally, a significant increase in oxygen
saturation following forest walking compared to city walking was also noted in patients
with hypertension [49].
Four studies investigated the impact of forest-based interventions on stress hormone
secretion. Serum cortisol and epinephrine significantly decreased in COPD patients after
forest bathing but not the city comparison [47]. Similarly, serum cortisol and urinary
adrenaline significantly decreased in participants with HNBP after forest therapy [54]. In
participants with HNBP or hypertension, there was a non-significant decrease in urinary
adrenaline and a significant decrease in urinary dopamine after forest walking compared
to urban walking, but both forest and urban walking significantly reduced urinary nora-
drenaline [53]. Salivary cortisol was also seen to reduce following forest intervention in
participants with hypertension [48,54].
Significant reductions of interferon-γ (IFN-γ), interleukin-6 (IL-6) and interleukin-8
(IL-8) were also reported after forest bathing but not the city comparison. In addition, slight
decreases in interleukin-1β (IL-1β), tumour necrosis factorα (TNF-α) and C-reactive protein
(CRP) were reported after forest bathing but not the city comparison [47]. Mao et al. [50,56]

Int. J. Environ. Res. Public Health 2022, 19, 3214
13 of 23
reported a significant decrease in IL-6 but no significant change in TNF-α after forest
bathing compared to controls. Mao et al. [50] also reported no significant change in
the high-sensitive-reactive protein, but a significant decrease in lipid peroxidation and a
significant increase in T-SOD after forest bathing compared to the comparison condition.
As Jia et al. [47] was the only study to investigate the impact of NBIs on patients with
COPD, they also assessed the impact of forest bathing on COPD-associated factors. These
included pulmonary and activation-regulated chemokine (PARC/CCL-18), surfactant pro-
tein D (SP-D) and tissue inhibitor of metalloproteinase (TIMP-1), as well as the proportion
of nature killer (NK) cells, nature killer T (NKT) cells and CD8+ T-lymphocytes, all of
which are associated with COPD exacerbations. They reported a significant decrease in
PARC/CCL-18 and TIMP-1 after forest bathing but not in the city group, and no significant
change in SP-D was reported in either group. There was a significant reduction of NK,
NKT-like and CD8+ T-cell expression of intracellular perforin after forest bathing but not
the city group, and no significant difference in the proportion of NK, NKT-like and CD8+
T-cells, nor their expression of granzyme B.
Two forest-based studies in patients with HNBP or hypertension also assessed specific
CVD-related factors. Li et al. [53] found a significant increase in serum adiponectin after
forest walking but not the urban comparison; no significant changes in other markers were
observed. Mao et al. [56] found a significant decrease in endothelin-1 and homocysteine,
RAS constituents including angiotensinogen, angiotensin II type 1 receptor (AT1), an-
giotensin II type 2 receptor (AT2), and a non-significant reduction in renin and angiotensin
II (Ang-II) after forest walking compared to controls. Blood pressure was poorly associated
with the change in renin, AT1 and angiotensinogen, while systolic blood pressure was
significantly related to Ang-II, endothelin-1 and homocysteine, and diastolic blood pressure
was significantly associated with Ang-II and endothelin-1. Similarly, patients with chronic
heart failure had a significant decrease in B-type natriuretic peptide (BNP) and endothelin-1
and a significant increase in angiotensin AT2 after forest walking compared to baseline [55];
no significant differences were observed from baseline in the control comparison groups.
There was no significant difference in N-terminal pro-BNP in either group.
3.4. Qualitative Participant Experiences
The only qualitative study identified in this review explored the experiences of
18 stroke survivors in late-phase recovery following a 12-week group horse-riding pro-
gramme [46]. Using semi-structured interviews, Pohl et al. [46] explored the impact of
the intervention on participants’ physical, psychological and social abilities; their general
mood; QoL; and their beliefs regarding the future. Four distinct themes were identified:
(1) transformative experiences, encompassing how the intervention altered participants’
view of themselves and their future; (2) human–horse interaction, involving the importance
of the physical and emotional relationship with the horse; (3) togetherness and belonging,
encompassing the significance of bonding with the group and instructors; (4) the all-in-one
solution, describing the richness of interactions with the horses, other group members
and staff.
3.5. Quality Appraisal
Using the BestBETS quality assessment checklist [39], the studies within this review
scored a range of 53–57 (mean = 50.4) out of a possible 64 points (see Supplementary Table S1).
All but one study [52] scored at least 75%, with four studies scoring over 80% [34,45,48,49].
The quality appraisal facilitated the identification of strengths and weaknesses of the
studies. Clear strengths included that all studies stated their aims and objectives, and
all had clear protocols reporting intervention and data collection. Seven studies were
randomised cohort studies, either having two between-groups comparisons or two cross-
over groups. Only two studies reported their randomisation methods [34,45], using an RCT
design and randomisation codes to allocate participants to each condition, respectively.

Int. J. Environ. Res. Public Health 2022, 19, 3214
14 of 23
Three out of twelve quantitative studies did not have a control comparison group
at baseline [52,54,55]. Song et al. [52,55] did not report a baseline group comparison,
although their cross-over design meant all participants completed both the forest and urban
sessions. Ochiai et al. [54], however, had no comparison group, limiting the strength of
their conclusions on the physiological and psychological effects of forest therapy, although
they did collect baseline measures prior to the intervention.
Li et al. [53] used a cross-over design that was not counterbalanced, meaning all
participants completed the urban session first followed but the forest session. Another
notable confound within this study was a 17 ◦C temperature difference between the two
conditions, with the highest temperature of 37 ◦C occurring on an urban day.
Additional limitations of the studies include an incomplete description of data, as all
but one study [56] reported only p-values, and none of the 12 quantitative studies mentioned
completing a priori calculations of power or effect size. Sample sizes were generally small,
with 11 studies reporting on fewer than 33 participants, often split into two comparison
groups so that group sizes did not exceed 23 participants, even with a 2:1 randomisation
ratio in favour of the intervention. Only Chun et al. [45] and Wichrowski et al. [51] reported
larger sample sizes of 59 and 107, respectively.
4. Discussion
The aim of this review was to systematically identify, appraise and synthesise pub-
lished evidence examining the impact of NBIs on psychological wellbeing of people living
with LTCs. Thirteen studies met inclusion criteria; twelve used quantitative methods and
one qualitative. All 13 studies reported a significant positive impact of NBIs on a range
of psychological wellbeing and physiological outcome measures and appear to support
previous research into NBIs’ benefits for physical and mental health, including two recent
reviews of forest-based interventions [19,43]. Quality appraisal of the studies showed mod-
est robustness and some methodological weaknesses, although there were four stronger
studies [34,45,48,49].
Positive psychological impacts of NBIs were particularly demonstrated within the
present review as eight of the 13 studies reported improvement on the most frequently used
measure, the POMS, following NBI in participants with HNBP or hypertension [47,49–54,56],
chronic heart failure [50], COPD [47], and those in cardiopulmonary rehabilitation [51]. The
most frequently used NBI intervention within this review was a forest-based intervention,
although the use of horticultural therapy also demonstrated significant psychological and
physiological improvements. The two studies to use horse-riding interventions [34,46] both
demonstrated positive psychological benefits, although they did not consider the potential
physiological impacts.
Positive physiological impacts of NBIs were also included, notably reporting reduced
blood pressure [49,54,56], which highlight NBIs’ potential impact to affect disease progres-
sion. Given hypertension’s well-established role in increasing risk of all-cause mortality,
cardiovascular and cerebrovascular events [66], and of CKD [67], any mitigation afforded
via its reduction can also reduce the risk of stroke [68], CVD events [69] and CVD in
individuals with T2DM [70].
That the review reveals positive physiological and psychological impacts of NBIs
for LTCs suggests parallels with benefits gained from interventions for LTCs, which cur-
rently have a more extensive evidence base. Exercise referral programmes, which are
often central to self-management of LTCs, also reduce blood pressure in general [71], as
well as in individuals with T2DM [72], and can mitigate hypertension, cholesterol and
diabetes [73]. A recent systematic review and meta-analysis of RCTs also concluded that
exercise interventions improved QoL in T2DM alongside physiological improvements [74],
findings previously evidenced for CVD and pulmonary diseases [75].
However, this review posits that the benefits of nature exposure through NBIs have
the potential to foster multilevel holistic benefits, exceeding that of exercise alone. For
example, Pohl et al. [46] identified positive impacts of a horse-riding intervention on partic-

Int. J. Environ. Res. Public Health 2022, 19, 3214
15 of 23
ipants’ physical, psychological and social abilities, general mood, QoL and future beliefs.
Notably, integral to NBIs is relaxation, reducing immediate stressors for the individual
and akin to other non-pharmacological interventions, such as massage, decreasing blood
pressure and HRV [76]. In this review, both heart rate and HRV were seen to improve
following NBIs [49,51,53,55], suggesting a relaxation effect akin to previous forest therapy
interventions that do not measure psychological outcomes [77,78]. Evidence for increased
relaxation is suggested by reduced stress hormone secretion, such as cortisol [79] and the
neurotransmitter dopamine [80]. This was replicated in the forest-based interventions
within this review, seeing decreases in cortisol [47,48,54], epinephrine [47], adrenaline [54]
and dopamine [53], in line with previous evidence that forest bathing lowers cortisol levels,
heart rate and blood pressure in individuals without chronic disease when compared to
city environments [77]. Studies within this review also evidenced inflammation reduction,
as well as reductions in COPD- and CVD-specific factors following NBIs. Inflammation is
linked to increased risk of CVD [81] and COPD [82], while repeated inflammation increase
is also associated with the development of depression [83]. Therefore, NBIs appear to have
a positive impact over and above the physiological gains.
Evidence for the benefit of NBIs in LTCs is in its infancy, yet recent research com-
paring forest bathing with compassion mind training in university students found that
the interventions had comparable psychological and physiological impacts [84]. This
shows promise since although McEwan and colleagues’ study is one of the first to offer
forest-based interventions within the UK, it is a much less familiar intervention within
the UK in comparison to its routine embedding in public health in Japan and other Asian
countries. Efficacy may not have been optimised given the UK’s cultural lack of familiarity
with the intervention and by its implementation in winter with cold weather a deterrent
to participation and with average air temperatures well below the minimum of 20 °C in
forest-based studies in our review.
Indeed, air temperature appears a confound for forest-based interventions within the
papers within our review and may moderate their effectiveness, particularly at extremes.
Given heat stress is a reported mortality risk factor [85], any higher temperatures may
have influenced the significant outcome differences reported, as might lower temperatures
since they are associated with higher blood pressure, especially in older adults [86]. Within
our review, Li et al. [53] did not observe higher blood pressure in their forest group in
line with the significantly lower temperature of that day and therefore concluded that the
forest intervention did decrease the blood pressure of participants, although this conclusion
should be considered with caution as their study design was not counterbalanced. Similarly,
Song et al. [52] also reported temperature difference between the forest and city days,
although they had counterbalanced their intervention order. In addition to the likelihood
of optimal conditions (including temperature and humidity), there are also individual
preferences at work, such that forest bathing on a warm (but not too hot) summer’s day
might have more appeal, and hence greater positive impact, than on a cold and wet winter’s
day. Such variation in individual temperature preferences and tolerances can depend on
regional and cultural norms.
Whilst showing the positive impacts of NBIs for LTCs, our findings cannot address
what works best, for whom and in what context [87]. The “active ingredients” of interven-
tions appear disparate. Horticultural therapy privileges hand–eye coordination, encourages
patients into the natural environment and relieves stress, but it also involves social inter-
action, gentle exercise and gaining satisfaction and a sense of purpose through work and
mastery through gaining new skills, well-researched constructs underpinning psychoso-
cial wellbeing. This is also reported following a care-farming programme for people in
rehabilitation from mental health problems [88]. Additionally, self-determination theory
suggests that those who have a vested interest gain more from the interventions, as seen in
exercise referral [89]. Forest-based approaches incorporate more physical movement and
exercise or meditation in a forest environment to promote wellbeing but can also involve
social interaction. Horse-riding interventions combine both exercise and social aspects,

Int. J. Environ. Res. Public Health 2022, 19, 3214
16 of 23
complemented by gaining satisfaction through overcoming a challenge and bond-building
with the animal [46]. This suggests that the social aspects of NBIs may be as important for
those with LTCs as are the nature elements. For example, CKD is also associated with social
isolation that is not solely due to poor mobility [90], and depression symptoms in patients
with CKD are associated with increased mortality [91] but can be reduced by building social
capital and support. Additionally, a systematic review of reviews and meta-analyses found
that peer-support improved clinical, behavioural, and psychological outcomes in patients
with T2DM [92], as well as QoL in patients with CKD [93]. Shame and stigma are commonly
experienced by people with LTCs, for example, in T2DM [94] and COPD [95]. Shame can
emanate from external stigma, e.g., from public or social perceptions, as well as internalised
self-stigma [96], making people feel isolated and powerless [97,98]. Perceived stigma is also
associated with psychological distress and less social support in T2DM [99] and COPD [100]
and negatively impacts medication adherence and help-seeking in COPD [100]. However,
by acknowledging vulnerability and reaching out to others, people are able to escape from
shame [97].
Nevertheless, psychosocial care of people with LTCs is rarely part of usual condition
management, often resulting in higher rates of GP appointments and unplanned primary
care admissions [101]. Social prescribing has been introduced in the UK to connect people
to emotional and practical support through community groups and statutory services to
improve their health and wellbeing [102]. More recently, social prescribing schemes have
been piloted by the UK government linking patients with green activities and NBIs [103].
Social prescribing link workers can help improve patient activation in 50+-year-olds with
one or more LTC [104]. Social prescribing reduces the number of GP appointments and
prescriptions [105], conveying economic benefits along with significant environmental and
social co-benefits [106,107]. Individual benefits of social prescriptions include improved
self-esteem, psychological wellbeing, sense of empowerment and self-management of LTCs,
as well as reduced anxiety, depression and isolation [108].
4.1. Clinical Implications
Given that LTCs have burgeoned, the psychological wellbeing of those with LTCs is
an important consideration in addition to the physiological impact on morbidity. Many
people with LTCs also have a mental health condition [109] or poorer mental health com-
pared to those without an LTC. For example, 40% of people with diabetes report poor
psychological wellbeing [110], and COPD is associated with poorer QoL [111]. This re-
view identified improvements in CVD-related factors within populations with HNBP or
hypertension, which are precursors for the development of CVD, as well as for stroke
and COPD. Providing NBIs alongside or shortly after diagnosis of LTCs may also address
multiple factors. For example, the reduction of disease progression and incidence of serious
outcomes, including premature mortality, as well as a method of symptom management as
they appear to improve psychological coping, which enables people to focus on specific
symptom management tasks required for their disease. This is an advantage of NBIs as,
since LTCs are defined by their lack of a cure, their use for such individuals would help not
only improve their psychological wellbeing but also, in turn, increase their ability to live a
fulfilling life that is less marred by their difficult symptoms. As of yet, there are no studies
published on the effects of NBIs in patients with diabetes, lung disease, liver disease or
CKD, which should be the focus of future research.
4.2. Strengths and Limitations
Studies within this review had some significant vulnerabilities, not least their limited
sample sizes and variation in participant culture, race and age. Most research has been
conducted in Asia, many studies by the same research group, raising concerns about
the generalisability of findings, as well as their cross-cultural relevance and application.
Although we sought out grey literature in our searches, publication bias may be evidenced
since reporting of significant results or positive effects are more likely to be published

Int. J. Environ. Res. Public Health 2022, 19, 3214
17 of 23
than non-significant results or negative effects [112]. Research into NBIs and LTCs is at a
germinal stage, which may further increase bias as small studies such as these are also more
likely to have positive bias. It is also important to understand the specific benefits of NBIs
for more vulnerable groups, particularly ethnic minorities, who have a higher prevalence
of LTCs [1], such as CKD [113]. Some argue this is due to the “weathering” hypothesis:
the premise that the increased stress experienced throughout the life course as a result of
structural racism leads to poorer outcomes [114].
Additionally, the validity of some of the studies is in question, particularly in relation
to the constraints placed on participants. Of note was that a number of the forest-based
studies did not permit participants to consume alcohol or caffeine or to smoke during
the interventions [49,50,53,55,56]. Whilst this may control for potential confounds, the
impacts of abstinence are unexamined. Notably, Ochiai et al. [54] did not permit participant
interaction during the programme or use of mobile phones. With no comparison group, the
impact they reported could be attributed to the restricted social interaction, either positively
or negatively.
4.3. Review Limitations
Limitations of the present review include the relatively small number of studies
included, which constrain comparison or firm conclusions. In addition, the majority of
studies reported p-values only; therefore, it was not possible to conduct a meta-analysis.
Nevertheless, a meta-analytic approach to compiling the effects of NBIs on LTCs is a
direction for future research that we would recommend. Our review included English
language papers only, yet elicited studies were conducted predominantly in Asia, in which
English is not a first language, which may have unintentionally excluded further studies.
The LTCs investigated were circumscribed and did not cover the widest range of
debilitating conditions. However, in constraining our focus to CVD, stroke, lung disease,
liver disease, CKD and T2DM, we were mindful of their population prevalence, morbidity
and mortality, and their impact on healthcare demand and patient QoL, as well as their
amenability to lifestyle and behavioural interventions for condition management [74,75].
Our review also highlighted absences in the literature thus far, notably the potential
barriers to implementing NBIs to enable their integration into mainstream healthcare
practice. Such barriers may include people being deterred from participation by aversions
to or fear of animals, the presence of allergies and access to transport. Depending on
the intervention, physical ability can be a barrier for many NBIs, as people with mobility
disabilities are less able to access greenspaces, even when they have the desire to [115].
Similarly, motivation or willingness to engage is likely to vary in relation to effort, for
example, a seven-day forest bathing trip compared to a 60-minute horticultural therapy
session in a hospital garden. This is where social capital, as mentioned above, could also
increase motivation. Individual differences in nature connectedness may also moderate
the impact of NBIs as those with more connection to nature may be more likely to get
greater benefits from such interventions [116]. This could be a possible moderator of
individual benefit from NBIs, assuming that greater nature connectedness would mean
greater positive impact, as those with a greater affinity with nature are more likely to gain
benefit from it.
Further limitations of the present review include the inability to answer the question
of “what NBIs work best for which LTC in what contexts”. This is largely due to the limited
number of studies covering a range of both LTCs and NBIs, but this gap in the evidence
warrants further research and this limitation is not constrained to the present review. For
example, Wilkie and Davinson [117] conducted a scoping review of the prevalence and
effectiveness of NBIs on health-related behaviours and outcomes, reporting that there is
little evidence for the long-term efficacy of NBIs. Effects were small but positive and were
assessed over a short period of time with no follow-up. These are also limitations of the
studies included in the present review. Future research should investigate the strength of
these effects over time. Wilkie and Davinson also suggest investigating if different delivery

Int. J. Environ. Res. Public Health 2022, 19, 3214
18 of 23
lengths of NBIs have an impact on dose–response and thus inform treatment plans for
“minimum duration for maximum benefit” (p. 7).
Additionally, it may be that different durations of NBIs may work better for those
with certain LTCs than for others. Similarly, different delivery methods may suit different
patient groups more so than others. For instance, virtual reality is a recent phenomenon
in NBIs and evidence suggests delivering virtual NBIs demonstrates similar effects on
psychological wellbeing to that of physical environments [118,119]. However, the caveat
is a need for further understanding of which components of these virtual and physical
environments are interacting with nature to achieve positive impacts on psychological
wellbeing. Nevertheless, the use of virtual environments within NBIs may address some
of the barriers to engagement discussed above, particularly for those who are unable to
access real-life nature environments. For example, a recent study that was interrupted by
the COVID-19 restrictions also found that conducting many elements of NBIs remotely also
had therapeutic gain for participants with depression [120].
5. Conclusions
The area of NBIs for LTCs shows limited but positive effects on a range of physical
health and psychological wellbeing outcomes, consistent with similar studies demonstrat-
ing the benefits of NBIs for health and wellbeing in other populations. Additional support
is provided by qualitative evidence that highlights the potentially transformative experi-
ences of participants. Future research could explore NBIs for other LTCs and incorporate
additional factors that may moderate effectiveness, such as nature connectedness.
Supplementary Materials: The following supporting information can be downloaded at: https:
//www.mdpi.com/article/10.3390/ijerph19063214/s1, Table S1: Quality assessment ratings using
BestBETS assessment checklist for cohort healthcare research.
Author Contributions: Conceptualization, E.M.T. and C.R.J.; methodology, E.M.T. and C.R.J.; val-
idation, E.M.T. and C.R.J.; formal analysis, E.M.T.; investigation, E.M.T.; writing—original draft
preparation, E.M.T.; writing—review and editing, C.R.J., N.R., C.J.L. and A.C.S.; supervision, C.R.J.
All authors have read and agreed to the published version of the manuscript.
Funding: A.C.S. and C.J.L. are grateful for research funding from the Stoneygate Trust. This report
is independent research supported by the National Institute for Health Research (NIHR) Leicester
Biomedical Research Centre. The views expressed are those of the authors and not necessarily those
of the Stoneygate Trust, National Health Service, NIHR or the Department of Health. This research
has received no other external funding.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Data Availability Statement: Not applicable.
Conflicts of Interest: The authors declare no conflict of interest.
References
1. KingsFund. Long-Term Conditions and Multi-Morbidity|The King’s Fund. Available online: https://www.kingsfund.org.
uk/projects/time-think-differently/trends-disease-and-disability-long-term-conditions-multi-morbidity (accessed on 31
January 2021).
2. British Medical Association. Prevention before Cure. Securing the Long-Term Sustainability of the NHS; BMA: London, UK, 2018.
3. Diabetes UK. Diabetes Prevalence 2019 | Diabetes UK. Available online: https://www.diabetes.org.uk/professionals/position-
statements-reports/statistics/diabetes-prevalence-2019 (accessed on 2 March 2021).
4. Kidney Care UK. Facts and Stats | Kidney Care UK. Available online: https://www.kidneycareuk.org/news-and-campaigns/
facts-and-stats/ (accessed on 12 February 2021).
5. Department of Health. Long Term Conditions Compendium of Information: Third Edition; Department of Health: London, UK, 2012.
6. Gascon, M.; Triguero-Mas, M.; Martinez, D.; Dadvand, P.; Rojas-Rueda, D.; Plasencia, A.; Nieuwenhuijsen, M.J. Residential green
spaces and mortality: A systematic review. Environ. Int. 2016, 86, 60–67. [CrossRef] [PubMed]

Int. J. Environ. Res. Public Health 2022, 19, 3214
19 of 23
7. Seo, S.; Choi, S.; Kim, K.; Kim, S.M.; Park, S.M. Association between urban green space and the risk of cardiovascular disease: A
longitudinal study in seven Korean metropolitan areas. Environ. Int. 2019, 125, 51–57. [CrossRef] [PubMed]
8. Bauwelinck, M.; Casas, L.; Nawrot, T.S.; Nemery, B.; Trabelsi, S.; Thomas, I.; Aerts, R.; Lefebvre, W.; Vanpoucke, C.; Van
Nieuwenhuyse, A.; et al. Residing in urban areas with higher green space is associated with lower mortality risk: A census-based
cohort study with ten years of follow-up. Environ. Int. 2021, 148, 106365. [CrossRef] [PubMed]
9. Paul, L.A.; Hystad, P.; Burnett, R.T.; Kwong, J.C.; Crouse, D.L.; van Donkelaar, A.; Tu, K.; Lavigne, E.; Copes, R.; Martin, R.V.; et al.
Urban green space and the risks of dementia and stroke. Environ. Res. 2020, 186, 109520. [CrossRef] [PubMed]
10. De la Fuente, F.; Saldías, M.A.; Cubillos, C.; Mery, G.; Carvajal, D.; Bowen, M.; Bertoglia, M.P. Green space exposure association
with type 2 diabetes mellitus, physical activity, and obesity: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 97.
[CrossRef] [PubMed]
11. Twohig-Bennett, C.; Jones, A. The health benefits of the great outdoors: A systematic review and meta-analysis of greenspace
exposure and health outcomes. Environ. Res. 2018, 166, 628–637. [CrossRef] [PubMed]
12. Ulrich, R.S. View through a window may influence recovery from surgery. Science 1984, 224, 420–421. [CrossRef]
13. Russell, R.; Guerry, A.D.; Balvanera, P.; Gould, R.K.; Basurto, X.; Chan, K.M.A.; Klain, S.; Levine, J.; Tam, J. Humans and nature:
How knowing and experiencing nature affect well-being. Annu. Rev. Environ. Resour. 2013, 38, 473–502. [CrossRef]
14. Kuo, M. How might contact with nature promote human health? Promising mechanisms and a possible central pathway.
Front. Psychol. 2015, 6, 1093. [CrossRef]
15. Wilson, E.O. Biophilia: The Human Bond with Other Species; Harvard University Press: Cambridge, MA, USA, 1984.
16. Myers, O.G. The Biophilia Hypothesis. Environ. Ethics 1996, 18, 327–330. [CrossRef]
17. Genter, C.; Roberts, A.; Richardson, J.; Sheaff, M. The contribution of allotment gardening to health and wellbeing: A systematic
review of the literature. Br. J. Occup. Ther. 2015, 78, 593–605. [CrossRef]
18. Cipriani, J.; Benz, A.; Holmgren, A.; Kinter, D.; McGarry, J.; Rufino, G. A Systematic Review of the Effects of Horticultural Therapy
on Persons with Mental Health Conditions. Occup. Ther. Ment. Health 2017, 33, 47–69. [CrossRef]
19. Wen, Y.; Yan, Q.; Pan, Y.; Gu, X.; Liu, Y. Medical empirical research on forest bathing (Shinrin-yoku): A systematic review.
Environ. Health Prev. Med. 2019, 24, 70. [CrossRef] [PubMed]
20. Li, Q.; Morimoto, K.; Nakadai, A.; Inagaki, H.; Katsumata, M.; Shimizu, T.; Hirata, Y.; Hirata, K.; Suzuki, H.; Miyazaki, Y.; et al.
Forest bathing enhances human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol.
2007, 20, 3–8. [CrossRef] [PubMed]
21. Li, Q.; Morimoto, K.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Suzuki, H.; Li, Y.J.; Wakayama, Y.;
et al. Visiting a forest, but not a city, increases human natural killer activity and expression of anti-cancer proteins. Int. J.
Immunopathol. Pharmacol. 2008, 21, 117–127. [CrossRef] [PubMed]
22. Tesler, R.; Endevelt, R.; Plaut, P. Urban Forest Health Intervention Program to promote physical activity, healthy eating, self-
efficacy and life satisfaction: Impact on Israeli at-risk youth. Health Promot. Int. 2021, daab145. [CrossRef] [PubMed]
23. Kabisch, N.; Püffel, C.; Masztalerz, O.; Hemmerling, J.; Kraemer, R. Physiological and psychological effects of visits to different
urban green and street environments in older people: A field experiment in a dense inner-city area. Landsc. Urban Plan. 2021,
207, 103998. [CrossRef]
24. Bailey, A.W.; Allen, G.; Herndon, J.; Demastus, C. Cognitive benefits of walking in natural versus built environments. World Leis. J.
2018, 60, 293–305. [CrossRef]
25. Berman, M.G.; Kross, E.; Krpan, K.M.; Askren, M.K.; Burson, A.; Deldin, P.J.; Kaplan, S.; Sherdell, L.; Gotlib, I.H.; Jonides, J.
Interacting with nature improves cognition and affect for individuals with depression. J. Affect. Disord. 2012, 140, 300–305.
[CrossRef]
26. Berget, B.; Braastad, B.O. Theoretical framework for animal-assisted interventions—Implications for practice. Ther. Communities
2008, 29, 323–337.
27. Beetz, A.; Uvnäs-Moberg, K.; Julius, H.; Kotrschal, K. Psychosocial and psychophysiological effects of human-animal interactions:
The possible role of oxytocin. Front. Psychol. 2012, 3, 234. [CrossRef] [PubMed]
28. Matchock, R.L. Pet ownership and physical health. Curr. Opin. Psychiatry 2015, 28, 386–392. [CrossRef] [PubMed]
29. Krause-Parello, C.A.; Gulick, E.E.; Basin, B. Loneliness, depression, and physical activity in older adults: The therapeutic role of
human–animal interactions. Anthrozoos 2019, 32, 239–254. [CrossRef]
30. McCune, S.; Kruger, K.A.; Griffin, J.A.; Esposito, L.; Freund, L.S.; Hurley, K.J.; Bures, R. Evolution of research into the mutual
benefits of human-animal interaction. Anim. Front. 2014, 4, 49–58. [CrossRef]
31. Sung, Y.-H.; Kim, C.-J.; Yu, B.-K.; Kim, K.-M. A hippotherapy simulator is effective to shift weight bearing toward the affected
side during gait in patients with stroke. NeuroRehabilitation 2013, 33, 407–412. [CrossRef]
32. Kim, Y.-N.; Lee, D.-K. Effects of horse-riding exercise on balance, gait, and activities of daily living in stroke patients. J. Phys.
Ther. Sci. 2015, 27, 607–609. [CrossRef]
33. Beinotti, F.; Correia, N.; Christofoletti, G.; Borges, G. Use of hippotherapy in gait training for hemiparetic post-stroke.
Arq. Neuropsiquiatr. 2010, 68, 908–913. [CrossRef]
34. Beinotti, F.; Christofoletti, G.; Correia, N.; Borges, G. Effects of horseback riding therapy on quality of life in patients post stroke.
Top. Stroke Rehabil. 2013, 20, 226–232. [CrossRef]

Int. J. Environ. Res. Public Health 2022, 19, 3214
20 of 23
35. Håkanson, M.; Möller, M.; Lindström, I.; Mattsson, B. The horse as the healer-A study of riding in patients with back pain.
J. Bodyw. Mov. Ther. 2009, 13, 43–52. [CrossRef]
36. Lundquist Wanneberg, P. Disability, Riding, and Identity: A Qualitative Study on the Influence of Riding on the Identity
Construction of People with Disabilities. Int. J. Disabil. Dev. Educ. 2014, 61, 67–79. [CrossRef]
37. Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for
clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [CrossRef] [PubMed]
38. Brooks, S.K.; Dunn, R.; Amlôt, R.; Greenberg, N.; James Rubin, G. Social and occupational factors associated with psychological
distress and disorder among disaster responders: A systematic review. BMC Psychol. 2016, 4, 18. [CrossRef] [PubMed]
39. BestBETs.org. BestBETs—BETs CA Worksheets. Available online: https://bestbets.org/links/BET-CA-worksheets.php (accessed
on 22 February 2021).
40. Marshall, C.; Sutton, A. Systematic Review Toolbox. Value Health 2016, 19, A398. [CrossRef]
41. Booth, A.; Sutton, A.; Papaioannou, D. Systematic Approaches to a Successful Literature Review, 2nd ed.; SAGE: London, UK, 2016;
ISBN 9780857021359.
42. Sandelowski, M.; Barroso, J. Reading Qualitative Studies. Int. J. Qual. Methods 2002, 1, 74–108. [CrossRef]
43. Stier-Jarmer, M.; Throner, V.; Kirschneck, M.; Immich, G.; Frisch, D.; Schuh, A. The Psychological and Physical Effects of Forests
on Human Health: A Systematic Review of Systematic Reviews and Meta-Analyses. Int. J. Environ. Res. Public Health 2021,
18, 1770. [CrossRef]
44. Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA
statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [CrossRef]
45. Chun, M.H.; Chang, M.C.; Lee, S.J. The effects of forest therapy on depression and anxiety in patients with chronic stroke.
Int. J. Neurosci. 2017, 127, 199–203. [CrossRef]
46. Pohl, P.; Carlsson, G.; Käll, L.B.; Nilsson, M.; Blomstrand, C. A qualitative exploration of post-acute stroke participants’ experiences
of a multimodal intervention incorporating horseback riding. PLoS ONE 2018, 13, e0203933. [CrossRef]
47. Jia, B.B.; Yang, Z.X.; Mao, G.X.; Lyu, Y.D.; Wen, X.L.; Xu, W.H.; Lyu, X.L.; Cao, Y.B.; Wang, G.F. Health Effect of Forest Bathing Trip
on Elderly Patients with Chronic Obstructive Pulmonary Disease. Biomed. Environ. Sci. 2016, 29, 212–218. [CrossRef]
48. Sung, J.; Woo, J.M.; Kim, W.; Lim, S.K.; Chung, E.J. The effect of cognitive behavior therapy-based “forest therapy” program on
blood pressure, salivary cortisol level, and quality of life in elderly hypertensive patients. Clin. Exp. Hypertens. 2012, 34, 1–7.
[CrossRef]
49. Wu, Q.; Ye, B.; Lv, X.; Mao, G.; Wang, S.; Chen, Z.; Wang, G. Adjunctive therapeutic effects of cinnamomum camphora forest
environment on elderly patients with hypertension. Int. J. Gerontol. 2020, 14, 327–331. [CrossRef]
50. Mao, G.; Cao, Y.; Wang, B.; Wang, S.; Chen, Z.; Wang, J.; Xing, W.; Ren, X.; Lv, X.; Dong, J.; et al. The Salutary Influence of Forest
Bathing on Elderly Patients with Chronic Heart Failure. Int. J. Environ. Res. Public Health 2017, 14, 368. [CrossRef] [PubMed]
51. Wichrowski, M.; Whiteson, J.; Haas, F.; Mola, A.; Rey, M.J. Effects of horticultural therapy on mood and heart rate in patients
participating in an inpatient cardiopulmonary rehabilitation program. J. Cardiopulm. Rehabil. 2005, 25, 270–274. [CrossRef]
[PubMed]
52. Song, C.; Ikei, H.; Kobayashi, M.; Miura, T.; Taue, M.; Kagawa, T.; Li, Q.; Kumeda, S.; Imai, M.; Miyazaki, Y. Effect of forest walking
on autonomic nervous system activity in middle-aged hypertensive individuals: A pilot study. Int. J. Environ. Res. Public Health
2015, 12, 2687–2699. [CrossRef]
53. Li, Q.; Kobayashi, M.; Kumeda, S.; Ochiai, T.; Miura, T.; Kagawa, T.; Imai, M.; Wang, Z.; Otsuka, T.; Kawada, T. Effects of Forest
Bathing on Cardiovascular and Metabolic Parameters in Middle-Aged Males. Evid.-Based Complement. Altern. Med. 2016, 2016,
2587381. [CrossRef]
54. Ochiai, H.; Ikei, H.; Song, C.; Kobayashi, M.; Takamatsu, A.; Miura, T.; Kagawa, T.; Li, Q.; Kumeda, S.; Imai, M.; et al.
Physiological and psychological effects of forest therapy on middle-aged males with high-normal blood pressure. Int. J. Environ.
Res. Public Health 2015, 12, 2532–2542. [CrossRef]
55. Song, C.; Ikei, H.; Kobayashi, M.; Miura, T.; Li, Q.; Kagawa, T.; Kumeda, S.; Imai, M.; Miyazaki, Y. Effects of viewing forest
landscape on middle-aged hypertensive men. Urban For. Urban Green. 2017, 21, 247–252. [CrossRef]
56. Mao, G.-X.; Cao, Y.-B.; Lan, X.-G.; He, Z.-H.; Chen, Z.-M.; Wang, Y.-Z.; Hu, X.-L.; Lv, Y.-D.; Wang, G.-F.; Yan, J. Therapeutic effect
of forest bathing on human hypertension in the elderly. J. Cardiol. 2012, 60, 495–502. [CrossRef]
57. Bunketorp-Käll, L.; Lundgren-Nilsson, Å.; Blomstrand, C.; Pekna, M.; Pekny, M.; Nilsson, M. The effects of a rhythm and
music-based therapy program and therapeutic riding in late recovery phase following stroke: A study protocol for a three-armed
randomized controlled trial. BMC Neurol. 2012, 12, 141. [CrossRef]
58. Bunketorp-Käll, L.; Pekna, M.; Pekny, M.; Blomstrand, C.; Nilsson, M. Effects of horse-riding therapy and rhythm and music-based
therapy on functional mobility in late phase after stroke. NeuroRehabilitation 2019, 45, 483–492. [CrossRef]
59. Pollock, V.; Cho, D.W.; Reker, D.; Volavka, J. Profile of Mood States: The Factors and Their Physiological Correlates. J. Nerv.
Ment. Dis. 1979, 167, 612–614. [CrossRef] [PubMed]
60. McHorney, C.A.; Ware, J.E.; Raczek, A.E. The MOS 36-item short-form health survey (Sf-36): II. Psychometric and clinical tests of
validity in measuring physical and mental health constructs. Med. Care 1993, 31, 247–263. [CrossRef] [PubMed]
61. Osgood, C.E.; Suci, G.J.; Tannenbaum, P. The Measurement of Meaning; University of Illinois Press: Urbana, IL, USA, 1957.

Int. J. Environ. Res. Public Health 2022, 19, 3214
21 of 23
62. Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients.
J. Personal. Assess. 1996, 67, 588–597. [CrossRef] [PubMed]
63. Hamilton, M. The Assessment of Anxiety States By Rating. Br. J. Med. Psychol. 1959, 32, 50–55. [CrossRef] [PubMed]
64. Barnes, L.L.B.; Harp, D.; Jung, W.S. Reliability Generalization of Scores on the Spielberger State-Trait Anxiety Inventory.
Educ. Psychol. Meas. 2002, 62, 603–618. [CrossRef]
65. Kim, K.Y.; Chun, B.Y.; Kam, S.; Lee, S.W.; Park, K.S.; Chae, S.C. Development of measurement scale for the quality of life in
hypertensive patients. J. Prev. Med. Public Health 2005, 38, 61–70.
66. Wang, Y.X.; Song, L.; Xing, A.J.; Gao, M.; Zhao, H.Y.; Li, C.H.; Zhao, H.L.; Chen, S.H.; Lu, C.Z.; Wu, S.L. Predictive Value of
Cumulative Blood Pressure for All-Cause Mortality and Cardiovascular Events. Sci. Rep. 2017, 7, 41969. [CrossRef]
67. Pugh, D.; Gallacher, P.J.; Dhaun, N. Management of Hypertension in Chronic Kidney Disease. Drugs 2019, 79, 365–379. [CrossRef]
68. Howard, G.; Banach, M.; Cushman, M.; Goff, D.C.; Howard, V.J.; Lackland, D.T.; McVay, J.; Meschia, J.F.; Muntner, P.;
Oparil, S.; et al. Is Blood Pressure Control for Stroke Prevention the Correct Goal?: The Lost Opportunity of Preventing Hyperten-
sion. Stroke 2015, 46, 1595–1600. [CrossRef]
69. Han, M.; Chen, Q.; Liu, L.; Li, Q.; Ren, Y.; Zhao, Y.; Liu, D.; Zhang, D.; Liu, F.; Chen, X.; et al. Stage 1 hypertension by the 2017
American College of Cardiology/American Heart Association hypertension guidelines and risk of cardiovascular disease events:
Systematic review, meta-analysis, and estimation of population etiologic fraction of prospective cohort studies. J. Hypertens. 2020,
38, 573–578. [CrossRef]
70. Sunkara, N.; Ahsan, H.C. Hypertension in diabetes and the risk of cardiovascular disease. Cardiovasc. Endocrinol. 2017, 6, 33–38.
[CrossRef] [PubMed]
71. Iwane, M.; Arita, M.; Tomimoto, S.; Satani, O.; Matsumoto, M.; Miyashita, K.; Nishio, I. Walking 10,000 steps/day or more reduces
blood pressure and sympathetic nerve activity in mild essential hypertension. Hypertens. Res. 2000, 23, 573–580. [CrossRef]
[PubMed]
72. Hayashino, Y.; Jackson, J.L.; Fukumori, N.; Nakamura, F.; Fukuhara, S. Effects of supervised exercise on lipid profiles and blood
pressure control in people with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Diabetes Res. Clin. Pract.
2012, 98, 349–360. [CrossRef] [PubMed]
73. Williams, P.T.; Thompson, P.D. Walking versus running for hypertension, cholesterol, and diabetes mellitus risk reduction.
Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1085–1091. [CrossRef]
74. Shah, S.Z.A.; Karam, J.A.; Zeb, A.; Ullah, R.; Shah, A.; Haq, I.U.; Ali, I.; Darain, H.; Chen, H. Movement is Improvement: The
Therapeutic Effects of Exercise and General Physical Activity on Glycemic Control in Patients with Type 2 Diabetes Mellitus: A
Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Ther. 2021, 12, 707–732. [CrossRef]
75. Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases.
Scand. J. Med. Sci. Sports 2015, 25, 1–72. [CrossRef]
76. Delaney, J.P.A.; Leong, K.S.; Watkins, A.; Brodie, D. The short-term effects of myofascial trigger point massage therapy on cardiac
autonomic tone in healthy subjects. J. Adv. Nurs. 2002, 37, 364–371. [CrossRef]
77. Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The physiological effects of Shinrin-yoku (taking in the forest
atmosphere or forest bathing): Evidence from field experiments in 24 forests across Japan. Environ. Health Prev. Med. 2010, 15,
18–26. [CrossRef]
78. Lee, J.; Tsunetsugu, Y.; Takayama, N.; Park, B.J.; Li, Q.; Song, C.; Komatsu, M.; Ikei, H.; Tyrväinen, L.; Kagawa, T.; et al. Influence
of forest therapy on cardiovascular relaxation in young adults. Evid.-Based Complement. Altern. Med. 2014, 2014, 834360. [CrossRef]
79. Dickerson, S.S.; Kemeny, M.E. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory
research. Psychol. Bull. 2004, 130, 355–391. [CrossRef]
80. Imperato, A.; Puglisi-Allegra, S.; Casolini, P.; Zocchi, A.; Angelucci, L. Stress-induced enhancement of dopamine and acetylcholine
release in limbic structures: Role of corticosterone. Eur. J. Pharmacol. 1989, 165, 337–338. [CrossRef]
81. Haraoui, B.; Liu, P.P.; Papp, K.A. Managing cardiovascular risk in patients with chronic inflammatory diseases. Clin. Rheumatol.
2012, 31, 585–594. [CrossRef] [PubMed]
82. Fairclough, L.; Urbanowicz, R.A.; Corne, J.; Lamb, J.R. Killer cells in chronic obstructive pulmonary disease. Clin. Sci. 2008, 114,
533–541. [CrossRef] [PubMed]
83. Bell, J.A.; Kivimäki, M.; Bullmore, E.T.; Steptoe, A.; Carvalho, L.A.; Vértes, P.E.; Cardinal, R.; Richardson, S.; Leday, G.; Freeman,
T.; et al. Repeated exposure to systemic inflammation and risk of new depressive symptoms among older adults. Transl. Psychiatry
2017, 7, e1208. [CrossRef] [PubMed]
84. McEwan, K.; Giles, D.; Clarke, F.J.; Kotera, Y.; Evans, G.; Terebenina, O.; Minou, L.; Teeling, C.; Basran, J.; Wood, W.; et al. A
pragmatic controlled trial of forest bathing compared with compassionate mind training in the UK: Impacts on self-reported
wellbeing and heart rate variability. Sustainability 2021, 13, 1380. [CrossRef]
85. Rainham, D.G.C.; Smoyer-Tomic, K.E. The role of air pollution in the relationship between a heat stress index and human
mortality in Toronto. Environ. Res. 2003, 93, 9–19. [CrossRef]
86. Woodhouse, P.R.; Khaw, K.; Plummer, M. Seasonal variation of blood pressure and its relationship to ambient temperature in an
elderly population. J. Hypertens. 1993, 11, 1267–1274. [CrossRef]

Int. J. Environ. Res. Public Health 2022, 19, 3214
22 of 23
87. Shanahan, D.; Astell–Burt, T.; Barber, E.; Brymer, E.; Cox, D.; Dean, J.; Depledge, M.; Fuller, R.; Hartig, T.; Irvine, K.; et al.
Nature–Based Interventions for Improving Health and Wellbeing: The Purpose, the People and the Outcomes. Sports 2019, 7, 141.
[CrossRef]
88. Ellingsen-Dalskau, L.H.; Morken, M.; Berget, B.; Pedersen, I. Autonomy support and need satisfaction in prevocational programs
on care farms: The self-determination theory perspective. Work 2016, 53, 73–85. [CrossRef]
89. Fortier, M.S.; Duda, J.L.; Guerin, E.; Teixeira, P.J. Promoting physical activity: Development and testing of self-determination
theory-based interventions. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 20. [CrossRef]
90. Moorthi, R.N.; Latham-Mintus, K. Social isolation in chronic kidney disease and the role of mobility limitation. Clin. Kidney J.
2019, 12, 602–610. [CrossRef] [PubMed]
91. Chilcot, J.; Guirguis, A.; Friedli, K.; Almond, M.; Day, C.; Da Silva-Gane, M.; Davenport, A.; Fineberg, N.A.; Spencer, B.;
Wellsted, D.; et al. Depression symptoms in haemodialysis patients predict all-cause mortality but not kidney transplantation: A
cause-specific outcome analysis. Ann. Behav. Med. 2018, 52, 1–8. [CrossRef] [PubMed]
92. Litchman, M.L.; Oser, T.K.; Hodgson, L.; Heyman, M.; Walker, H.R.; Deroze, P.; Rinker, J.; Warshaw, H. In-Person and Technology-
Mediated Peer Support in Diabetes Care: A Systematic Review of Reviews and Gap Analysis. Diabetes Educ. 2020, 46, 230–241.
[CrossRef] [PubMed]
93. Ghahramani, N.; Chinchilli, V.M.; Kraschnewski, J.L.; Lengerich, E.J.; Sciamanna, C.N. Effect of Peer Mentoring on Quality of Life
among CKD Patients: Randomized Controlled Trial. Kidney Dis. 2021, 7, 323–334. [CrossRef]
94. Basinger, E.D.; Farris, M.; Delaney, A.L. Investigating the Experience of Diabetes Stigma in Online Forums. South. Commun. J.
2019, 85, 43–57. [CrossRef]
95. Jerpseth, H.; Knutsen, I.R.; Jensen, K.T.; Halvorsen, K. Mirror of shame: Patients experiences of late-stage COPD. A qualitative
study. J. Clin. Nurs. 2021, 30, 2854–2862. [CrossRef]
96. Kato, A.; Fujimaki, Y.; Fujimori, S.; Izumida, Y.; Suzuki, R.; Ueki, K.; Kadowaki, T.; Hashimoto, H. A qualitative study on the
impact of internalized stigma on type 2 diabetes self-management. Patient Educ. Couns. 2016, 99, 1233–1239. [CrossRef]
97. Brown, B. Shame resilience theory: A grounded theory study on women and shame. Fam. Soc. 2006, 87, 43–52. [CrossRef]
98. Dayal, H.; Weaver, K.; Domene, J.F. From shame to shame resilience: Narratives of counselor trainees with eating issues.
Qual. Health Res. 2015, 25, 153–167. [CrossRef]
99. Gredig, D.; Bartelsen-Raemy, A. Diabetes-related stigma affects the quality of life of people living with diabetes mellitus in
Switzerland: Implications for healthcare providers. Health Soc. Care Community 2017, 25, 1620–1633. [CrossRef]
100. Woo, S.; Zhou, W.; Larson, J.L. Stigma experiences in people with chronic obstructive pulmonary disease: An integrative review.
Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 1647–1659. [CrossRef] [PubMed]
101. Cruwys, T.; Wakefield, J.R.H.; Sani, F.; Dingle, G.A.; Jetten, J. Social Isolation Predicts Frequent Attendance in Primary Care.
Ann. Behav. Med. 2018, 52, 817–829. [CrossRef] [PubMed]
102. Bickerdike, L.; Booth, A.; Wilson, P.M.; Farley, K.; Wright, K. Social prescribing: Less rhetoric and more reality. A systematic
review of the evidence. BMJ Open 2017, 7, e013384. [CrossRef]
103. NHS. NHS England » Social Prescribing. Available online: https://www.england.nhs.uk/personalisedcare/social-prescribing/
green-social-prescribing/ (accessed on 16 January 2022).
104. Elston, J.; Gradinger, F.; Asthana, S.; Lilley-Woolnough, C.; Wroe, S.; Harman, H.; Byng, R. Does a social prescribing ‘holistic’
link-worker for older people with complex, multimorbidity improve well-being and frailty and reduce health and social care use
and costs? A 12-month before-and-after evaluation. Prim. Health Care Res. Dev. 2019, 20, e135. [CrossRef] [PubMed]
105. Lynch, M.; Jones, C. Social prescribing for frequent attenders: Findings from an innovative pilot intervention. Lancet 2019,
394, S69. [CrossRef]
106. Bloomfield, D. What makes nature-based interventions for mental health successful? BJPsych. Int. 2017, 14, 82–85. [CrossRef]
[PubMed]
107. Schoen, V.; Caputo, S.; Blythe, C. Valuing physical and social output: A rapid assessment of a London community garden.
Sustainability 2020, 12, 5452. [CrossRef]
108. Carrier, J.; Newbury, G. Managing long-term conditions in primary and community care. Br. J. Community Nurs. 2016, 21, 504–508.
[CrossRef]
109. Naylor, C.; Parsonage, M.; Mcdaid, D.; Knapp, M.; Fossey, M.; Galea, A. Long-Term Condition and Mental Health: The Cost of
Co-Morbidities; King’s Fund: London, UK, 2012; pp. 1–32.
110. Whicher, C.A.; O’Neill, S.; Holt, R.I.G. Diabetes in the UK: 2019. Diabet. Med. 2020, 37, 242–247. [CrossRef]
111. Hurst, J.R.; Skolnik, N.; Hansen, G.J.; Anzueto, A.; Donaldson, G.C.; Dransfield, M.T.; Varghese, P. Understanding the impact
of chronic obstructive pulmonary disease exacerbations on patient health and quality of life. Eur. J. Intern. Med. 2020, 73, 1–6.
[CrossRef]
112. Song, F.; Parekh, S.; Hooper, L.; Loke, Y.K.; Ryder, J.; Sutton, A.J.; Hing, C.; Kwok, C.S.; Pang, C.; Harvey, I. Health Technology
Assessment NIHR HTA programme www.hta.ac.uk Dissemination and publication of research findings: An updated review of
related biases. Health Technol. Assess. 2010, 14. [CrossRef] [PubMed]
113. Weldegiorgis, M.; Smith, M.; Herrington, W.G.; Bankhead, C.; Woodward, M. Socioeconomic disadvantage and the risk of
advanced chronic kidney disease: Results from a cohort study with 1.4 million participants. Nephrol. Dial. Transplant. 2020, 35,
1562–1570. [CrossRef] [PubMed]

Int. J. Environ. Res. Public Health 2022, 19, 3214
23 of 23
114. Geronimus, A.T.; Hicken, M.; Keene, D.; Bound, J. “Weathering” and age patterns of allostatic load scores among blacks and
whites in the United States. Am. J. Public Health 2006, 96, 826–833. [CrossRef] [PubMed]
115. Corazon, S.S.; Gramkow, M.C.; Poulsen, D.V.; Linlygum, V.; Zhang, G.; Stigsdotter, U.K. I would really like to visit the forest, but
it is just too difficult: A qualitative study on mobility disability and green spaces. Scand. J. Disabil. Res. 2019, 21, 1–13. [CrossRef]
116. Mayer, F.S.; Frantz, C.M.P. The connectedness to nature scale: A measure of individuals’ feeling in community with nature.
J. Environ. Psychol. 2004, 24, 503–515. [CrossRef]
117. Wilkie, S.; Davinson, N. Prevalence and effectiveness of nature-based interventions to impact adult health-related behaviours and
outcomes: A scoping review. Landsc. Urban Plan. 2021, 214, 104166. [CrossRef]
118. Mattila, O.; Korhonen, A.; Pöyry, E.; Hauru, K.; Holopainen, J.; Parvinen, P. Restoration in a virtual reality forest environment.
Comput. Human Behav. 2020, 107, 106295. [CrossRef]
119. Yu, C.P.; Lee, H.Y.; Luo, X.Y. The effect of virtual reality forest and urban environments on physiological and psychological
responses. Urban For. Urban Green. 2018, 35, 106–114. [CrossRef]
120. Salonen, K.; Hyvönen, K.; Paakkolanvaara, J.-V.; Korpela, K. Flow With Nature Treatment for Depression: Participants’ Experi-
ences. Front. Psychol. 2022, 12, 768372. [CrossRef]
