
Hindawi Publishing Corporation
Evidence-Based Complementary and Alternative Medicine
Volume 2015, Article ID 671094, 7 pages
http://dx.doi.org/10.1155/2015/671094
Research Article
Analysis of Individual Variations in Autonomic Responses to
Urban and Forest Environments
Hiromitsu Kobayashi,1 Chorong Song,2 Harumi Ikei,2,3
Takahide Kagawa,3 and Yoshifumi Miyazaki2
1Ishikawa Prefectural Nursing University, 1-1 Gakuendai, Kahoku, Ishikawa 929-1210, Japan
2Center for Environment, Health and Field Sciences, Chiba University, 6-2-1 Kashiwa-no-ha, Kashiwa-shi, Chiba 277-0882, Japan
3Forestry and Forest Products Research Institute, 1 Matsunosato, Tsukuba, Ibaraki 305-8687, Japan
Correspondence should be addressed to Yoshifumi Miyazaki; ymiyazaki@faculty.chiba-u.jp
Received 29 July 2015; Accepted 20 September 2015
Academic Editor: Giuseppe Caminiti
Copyright © 2015 Hiromitsu Kobayashi et al. This is an open access article distributed under the Creative Commons Attribution
License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly
cited.
Autonomic responses to urban and forest environments were studied in 625 young male subjects. The experimental sites were 57
forests and 57 urban areas across Japan. The subjects viewed the landscape (forest or urban environment) for a period of 15 min
while sitting on a chair. During this period, heart rate variability (HRV) was monitored continuously. The results were presented as
histograms and analyzed with special reference to individual variations. Approximately 80% of the subjects showed an increase in
the parasympathetic indicator of HRV (lnHF), whereas the remaining subjects showed a decrease in the parasympathetic activity.
Similarly, 64.0% of the subjects exhibited decreases in the sympathetic indicator of HRV (ln[LF/HF]), whereas the remaining
subjects showed opposite responses. Analysis of the distribution of HRV indices (lnHF and ln[LF/HF]) demonstrated the effect
of forest environments on autonomic activity more specifically than the conventional analysis based on the difference in mean
values.
1. Introduction
Recently, there has been growing interest in the effects of the
natural environment on human health. Beneficial effects may
include stress relief, improved cognition and physical activity,
better social cohesion, and promotion of overall health and
mental well-being [1]. The predisposition of humans to
responding positively to the natural environment may be a
result of past adaptation to natural environments for survival
or ongoing well-being during evolution [2].
The psychological effects of exposure to a forest envi-
ronment on emotions have been demonstrated by various
researchers. Bowler et al. [3] performed a meta-analysis of the
results of several studies on the effect of natural environments
and concluded that the natural environment has a consistent
effect of reducing negative emotions (anger, fatigue, or sad-
ness). In addition, exposure to a forest environment may have
a positive effect on psychiatric impairments, such as alcoholic
depression [4].
In recent years, along with psychological responses,
physiological responses to a forest environment have been
investigated. Studies have demonstrated that exposure to a
forest environment results in reduced physiological indi-
cators for stress. For example, lower fluctuation in skin
conductance, shorter pulse-transit time (suggesting lower
blood pressure), lower tension in frontalis muscles, and lower
heart rate were observed during exposure to a video of natural
settings [2]. Exposure to real forest environments decreased
salivary cortisol concentration [5, 6] and cerebral blood flow
(indicating a relaxation in brain activity) [6] and increased
natural killer (NK) cells (indicating an enhancement of the
immune system) [7–9]. Furthermore, Ohtsuka et al. [10]
reported that a long-term experience in forest environment
has significantly reduced blood glucose levels in patients with
diabetes.
The current study investigated the effects of forest envi-
ronments on autonomic nervous activity using heart rate
variability (HRV) as an indicator. The relationship between

2
Evidence-Based Complementary and Alternative Medicine
HRV and autonomic functions has been established by
previous studies [11–13]. Use of HRV as a physiological
indicator of stress is also well established. In addition, during
recent years within the field of alternative medicine, the effect
of acupuncture has been evaluated by HRV [14, 15].
Our previous studies have demonstrated an increase in
the high frequency (HF) component and/or a decrease in the
low frequency (LF)/HF ratio of HRV in forest environments
[16–19]. Similar results have been observed in parks in urban
areas [20, 21] or during exposure to indoor plants [22, 23]. The
HF component of HRV is considered a marker of parasym-
pathetic activity, whereas the LF component or LF/HF ratio
is considered a marker of sympathetic activity [24]. Thus,
the results of the abovementioned studies suggested a relative
activation of the parasympathetic function.
Use of HRV for evaluating stress provides some advan-
tages over alternative physiological measurements. HRV can
be recorded continuously in a noninvasive manner. Fur-
thermore, use of a portable heart rate monitor can provide
ambulatory recording of HRV. These advantages might be
maximized in field studies rather than experimental studies.
Thus, HRV can be the appropriate indicator of physiological
responses to forest environments.
One of the features of the current study is an analysis
with special reference to the distribution characteristics of
individual variations in the HRV response. Most previous
studies on physiological responses to environments have
focused on the differences in the mean value; individual
variations were considered an error or impurities. Individual
variations have been an underutilized resource in various
fields of life sciences. Bennett [25] described the tendency of
focusing on means as the “tyranny of the golden mean.” From
the viewpoint of adaptation, individual variations should
have biological and/or evolutionary significance and should
decidedly not be viewed as an error or impurity. The mean
value has certain significance as one of the representative
values of a population; however, it represents no more signif-
icance than any other aspect of the physiological responses of
a population.
Analysis of the physiological response focusing on indi-
vidual variations may be challenging compared with an
analysis focusing on the mean value because the analysis
of individual variations requires a larger sample size. The
current study investigated the frequency components of HRV
in 625 young Japanese males in urban and forest environ-
ments. The current study utilized a large sample size and
can therefore provide a new perspective on the physiological
responses to natural environments.
2. Materials and Methods
2.1. Study Sites and Subjects. The study sites were 57 forest
and 57 urban areas across Japan. The chosen urban sites were
downtown or nearby a Japan Railway (JR) station.
Although 684 young Japanese male university students
participated in the experiments, only 625 subjects with
complete data for both urban and forest sites were included
in the analysis. The demographic parameters of the subjects
are shown in Table 1.
Table 1: Demographic parameters of the subjects (𝑛 = 625).
Age (years)
Mean
21.6
SD
1.6
Max
29
Min
19
∗SD: standard deviation.
Height (cm)
172.3
5.6
188
155
Weight (kg)
64.7
9.6
110
42
None of the subjects reported a history of physical or
psychiatric disorders. Consumption of alcohol and tobacco
was prohibited and consumption of caffeine was controlled
during the study period. The study was performed according
to the regulations of the Ethics Committee of the Center for
Environment, Health, and Field Sciences, Chiba University,
or the Institutional Ethics Committee of the Forestry and
Forest Products Research Institute in Japan.
2.2. Physiological Measurements. HRV was measured using
a portable electrocardiograph (Activtracer AC-301A, GMS,
Japan). Spectral analysis of HRV was conducted for 15
min recordings using HRV software (MemCalc/Win, GMS,
Tokyo, Japan) based on the maximum entropy method. The
HF and LF components were obtained by integration of
the power spectra at the respective ranges of 0.15–0.40 and
0.04–0.15 Hz. The natural logarithms of the HRV indices
(lnHF, ln[LF/HF]) were then calculated, considering that the
raw HRV components indicate skewed distributions [26]. In
the current study, HRV was measured during spontaneous
breathing, and paced breathing was not applied. The subjects
were instructed to avoid irregular breathing during the
measurements. A previous study has reported that paced
breathing has a negligible effect on interindividual variations
in the spectral components of HRV [27].
2.3. Experimental Design. The experiment was performed at
each experimental site over 2 consecutive days. Before the
experiment, the aim of the study and the experimental pro-
tocol was explained and general instructions were provided
to the subjects. The subjects ate lunch between 11:30 and
12:30, and the measurements were conducted between 13:30
and 15:30. All subjects were nonsmokers. Alcohol intake and
unusual physical activity on the day before the measurement
were forbidden.
The subjects at each site were randomly divided into two
groups, and the order of exposure to the experimental con-
ditions (urban or forest) differed among the two groups. One
group was exposed to the forest site prior to the urban site,
and the other group followed the reverse order. All subjects
remained in a waiting room before moving to the field site.
All subjects were instructed to rest on a chair for 5 min, which
mitigated the physiological effects of any possible physical
activity before the measurement period. HRV measurements
were obtained during 15 min when the subjects viewed the
landscape. On the second day, the subjects switched the field
sites. The experimental protocol on the second day was the
same as that on the first day.

Evidence-Based Complementary and Alternative Medicine
3
Table 2: Descriptive statistics of the distribution of heart rate variability (𝑛 = 625).
ln HF
ln(LF/HF)
Urban
Forest
Urban-forest
Urban
Forest
Urban-forest
Mean
5.54
6.02
−0.48
1.48
1.32
0.16
Median
5.66
6.14
−0.45
1.51
1.31
0.16
SD
0.92
1.01
0.70
0.77
0.82
0.71
5th percentile
3.81
4.41
−1.57
0.16
0.00
−0.92
95th percentile
6.9
7.29
0.56
2.70
2.66
1.19
Skewness
−1.20
−1.10
−0.92
−0.21
0.32
−0.46
Kurtosis
3.42
2.97
8.21
0.47
0.97
4.24
∗SD: standard deviation; skewness: a measure of symmetry of distribution; Kurtosis: a measure of whether the distribution curve is peaked (positive) or flat
(negative) relative to the normal distribution.
Among the experiments at 57 sites, the experimental
design used at 44 sites was the “Stay-in Forest Therapy”
design, where arrangements were made for all subjects to
reside in a hotel with identical single rooms. At the remaining
13 sites, the experimental design of “One-Day Forest Therapy”
was used, where the subjects were allowed to return home
after the first day. To reduce inconvenience to subjects and
to limit research expenses, we switched to the simplified
experimental design of One-Day Forest Therapy.
2.4. Data Analysis. Mean, median, standard deviation (SD),
5th and 95th percentile values, skewness, and kurtosis were
calculated for each HRV index. Skewness is a measure of
symmetry of distribution. Negative or positive skewness is
indicated when the left or right tail of the research data
fitted to a histogram is longer, respectively. The skewness of a
normal distribution is zero. Kurtosis is a measure of whether
the distribution curve is peaked (positive) or flat (negative)
relative to the normal distribution. The kurtosis of a normally
distributed data set is zero. Statistical analysis was performed
using IBM SPSS statistics ver. 21 (IBM, New York, US).
3. Results and Discussion
3.1. Individual Variations in Autonomic Responses to a Forest
Environment. The descriptive statistics of the distribution
of the HRV indices are summarized in Table 2. The mean
lnHF in urban and forest environments was 5.54 and 6.02
[ln(ms2)], respectively. A larger lnHF value was observed in
forest environments, suggesting activation of the parasym-
pathetic function. The histograms for lnHF are shown in
Figure 1. Although the mean values were different, the dis-
tribution curves were almost identical between urban and
forest environments. In both environments, the distributions
indicated slightly left skewed and peaked curves compared
with the normal distribution (Figures 1(a) and 1(b)). Dif-
ferences in lnHF between urban and forest environments
were calculated individually and depicted as a histogram
(Figure 1(c)). Negative values in the difference imply that
lnHF was larger in forest than in urban environments,
whereas the positive values in the difference imply that
lnHF was smaller in forest than in urban environments. A
histogram showing the difference indicated a slightly left-
skewed and markedly peaked distribution. In the results of
the current study, 495 out of 625 (79.2%) subjects exhibited
an increase in lnHF in forest environments.
The results of ln(LF/HF) were analyzed in a similar way to
lnHF. The mean ln(LF/HF) in urban and forest environments
was 1.48 and 1.32, respectively. Lower ln(LF/HF) values were
observed in forest environments, suggesting suppression of
the sympathetic function. The histograms for ln(LF/HF)
are shown in Figure 2. A histogram for the difference in
ln(LF/HF) between urban and forest environments indicated
an almost symmetrical distribution (Figures 2(a) and 2(b)).
Furthermore, 397 out of 625 (63.5%) subjects exhibited a
decrease in ln(LF/HF) in forest environments (Figure 2(c)).
In summary, approximately 80% of the subjects showed
an increase in the parasympathetic activity in forest environ-
ments, whereas the remaining subjects exhibited a negative
effect of the forest environments. The mean lnHF in urban
and forest environments was 5.54 and 6.02 [ln(ms2)], respec-
tively; thus, the change was approximately 9%. Although the
difference in the mean values was statistically significant,
the difference appeared marginal. On the other hand, the
ratio of positive and negative responders (80 : 20) was a more
conclusive result as compared with the changes in the mean
values. Similar results were obtained for the sympathetic
indicator [ln(LF/HF)]. Thus, histograms are considered to be
an efficient tool for the analysis of physiological responses
to natural environments in which larger individual variation
exists.
3.2. Effect of Respiration and Air Pollution. It has been
known that HRV is closely related with respiratory rate.
Slower respiratory rate produces larger HF power in the
HRV spectrum. Increased HF in a forest environment may
relate with slower and/or deeper respiration. Gladwell et al.
[28] studied the effects of natural and urban landscapes on
heart rate, blood pressure, and respiration. In their results,
no significant changes were observed in the respiratory rate
and depth, although a parasympathetic indicator of HRV
increased in natural landscape similar to the present results.
Thus, the change in the respiratory rate is not considered to
be a major cause of the increased HF in forest.
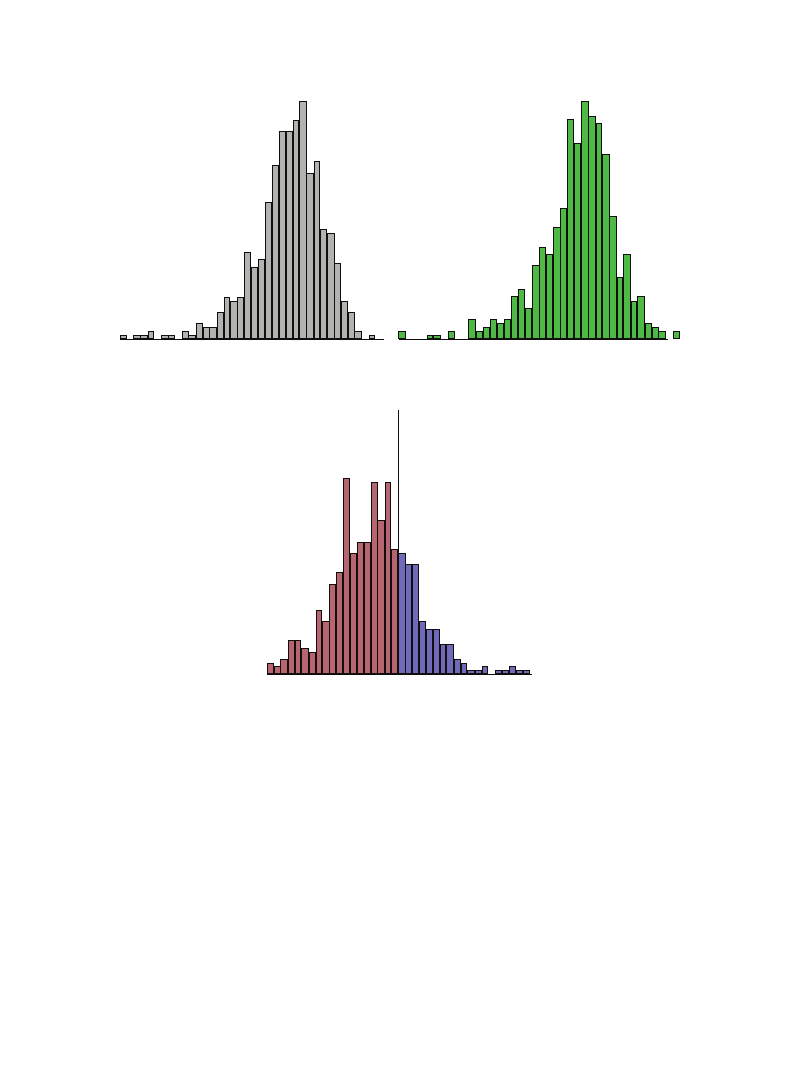
4
lnHF
Evidence-Based Complementary and Alternative Medicine
(a) Urban environment
Increase
at forest site
79.2%
(b) Forest environment
0
Decrease
at forest site
20.8%
(c) Difference (urban-forest)
Figure 1: Histograms showing the high frequency component (lnHF) of heart rate variability in urban and forest environments. (a) lnHF at
urban sites, (b) lnHF at forest sites, and (c) difference in lnHF between urban and forest sites.
In recent years, the effect of air pollution on human HRV
has been attracting attention in the field of environmental
medicine. A relationship between increased PM2.5 (par-
ticulate matter with an aerodynamic diameter of <2.5 𝜇m)
and decreased parasympathetic indicator of HRV has been
reported [29, 30]. Because the effect of PM2.5 was considered
to be acute rather than chronic [31], the difference in air
pollution between urban and forest environments may be an
explanation of the present results.
3.3. Biophilia and Biophobia. The biophilia hypothesis was
proposed by the distinguished biologist Wilson [32]. Bio-
philia is defined as the “innate tendency to focus on life and
life-like processes” [33]. This tendency could be explained
from an evolutionary perspective. For millions of years,
human beings (or their recent ancestors) lived in the savannas
of Africa. Within this environment, natural features such as
trees or forests could provide food, water, or shelter, thereby
increasing the probability of survival. Thus, biophilia can be
regarded as an adaptive characteristic of human evolution.
On the other hand, it has been known that certain people
show a strong dislike for natural settings. This tendency is
called biophobia [34]. Biophobia includes certain specific
phobias, such as arachnophobia (irrational fear of spiders)
or entomophobia (fear of insects). Previous studies have
reported that patients displaying arachnophobia showed
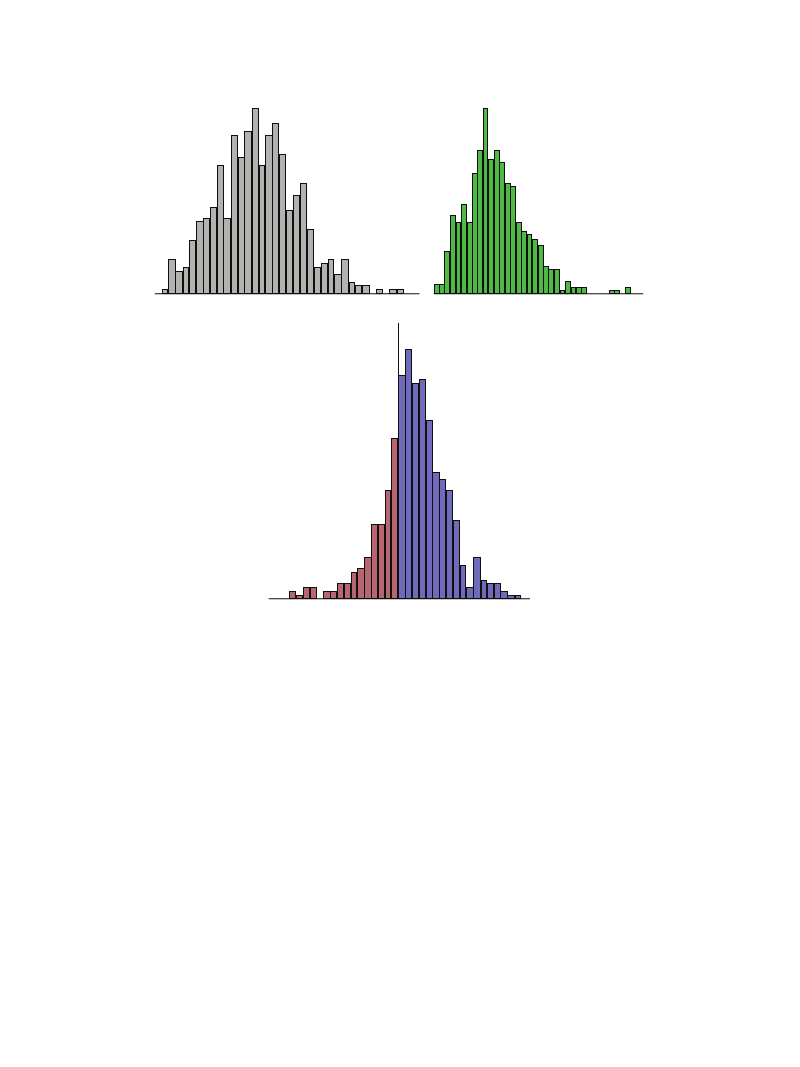
Evidence-Based Complementary and Alternative Medicine
5
ln(LF/HF)
(a) Urban environment
Increase
at forest site
36.5%
(b) Forest environment
Decrease
at forest site
63.5%
(c) Difference (urban-forest)
Figure 2: Histograms showing the low frequency/high frequency ratio (ln[LF/HF]) of heart rate variability in urban and forest environments.
(a) ln(LF/HF) at urban sites, (b) ln(LF/HF) at forest sites, and (c) difference in ln(LF/HF) between urban and forest sites.
increased HR or HRV during presentation of images of spi-
ders [35, 36]. Without actually perceiving spiders or insects,
people exhibiting these phobias manifested phobic reactions
even if they merely imagined spiders or insects in their
immediate environment. Therefore, the negative responders
in the results of the current study could be explained by these
specific phobias to living things. Further consideration of
the relationship between physiological responses to the forest
environment and biophobia is expected in future studies.
4. Conclusion
Autonomic responses to a forest environment were studied
in 625 young male subjects. The results were demonstrated
using histograms and were analyzed with special reference
to individual variations. An increase in the parasympathetic
indicator of HRV (lnHF) was observed in approximately
80% of the subjects. Analysis of the distribution of HRV
indices (lnHF and ln[LF/HF]) demonstrated the effect of
forest environments on autonomic activity more specifically
than the conventional analysis based on mean values.
Conflict of Interests
The authors declare that there is no conflict of interests
regarding the publication of this paper.
Acknowledgments
The authors thank Dr. Yuko Tsunetsugu, Mr. Takeshi
Morikawa of Forestry and Forest Products Research Institute,
Dr. Bum-Jin Park of Chungnam National University, and Dr.
Juyoung Lee of Korea Forest Service for their assistance in
the experiments. This study was supported by the 2015 Strate-
gic Innovation Promotion (SIP) Program of the National
Agriculture and Food Research Organization (NARO) in
Japan.

6
Evidence-Based Complementary and Alternative Medicine
References
[1] A. C. Logan, “Dysbiotic drift: mental health, environmental
grey space, and microbiota,” Journal of Physiological Anthropol-
ogy, vol. 34, no. 1, article 23, 2015.
[2] R. S. Ulrich, R. F. Simons, B. D. Losito, E. Fiorito, M. A. Miles,
and M. Zelson, “Stress recovery during exposure to natural and
urban environments,” Journal of Environmental Psychology, vol.
11, no. 3, pp. 201–230, 1991.
[3] D. E. Bowler, L. M. Buyung-Ali, T. M. Knight, and A. S. Pullin,
“A systematic review of evidence for the added benefits to health
of exposure to natural environments,” BMC Public Health, vol.
10, no. 1, article 456, 2010.
[4] W. S. Shin, C. S. Shin, and P. S. Yeoun, “The influence of
forest therapy camp on depression in alcoholics,” Environmental
Health and Preventive Medicine, vol. 17, no. 1, pp. 73–76, 2012.
[5] J. Sung, J.-M. Woo, W. Kim, S.-K. Lim, and E.-J. Chung,
“The effect of cognitive behavior therapy-based ‘forest therapy’
program on blood pressure, salivary cortisol level, and quality of
life in elderly hypertensive patients,” Clinical and Experimental
Hypertension, vol. 34, no. 1, pp. 1–7, 2012.
[6] B.-J. Park, Y. Tsunetsugu, T. Kasetani et al., “Physiological effects
of Shinrin-yoku (taking in the atmosphere of the forest)—using
salivary cortisol and cerebral activity as indicators,” Journal of
Physiological Anthropology, vol. 26, no. 2, pp. 123–128, 2007.
[7] Q. Li, K. Morimoto, M. Kobayashi et al., “A forest bathing
trip increases human natural killer activity and expression of
anti-cancer proteins in female subjects,” Journal of Biological
Regulators & Homeostatic Agents, vol. 22, no. 1, pp. 45–55, 2008.
[8] Q. Li, M. Kobayashi, Y. Wakayama et al., “Effect of phytoncide
from trees on human natural killer function,” International
Journal of Immunopathology and Pharmacology, vol. 22, no. 4,
pp. 951–959, 2009.
[9] Q. Li, “Effect of forest bathing trips on human immune
function,” Environmental Health and Preventive Medicine, vol.
15, no. 1, pp. 9–17, 2010.
[10] Y. Ohtsuka, N. Yabunaka, and S. Takayama, “Shinrin-yoku
(forest-air bathing and walking) effectively decreases blood
glucose levels in diabetic patients,” International Journal of
Biometeorology, vol. 41, no. 3, pp. 125–127, 1998.
[11] S. Akselrod, D. Gordon, F. A. Ubel, D. C. Shannon, A. C.
Berger, and R. J. Cohen, “Power spectrum analysis of heart rate
fluctuation: a quantitative probe of beat-to-beat cardiovascular
control,” Science, vol. 213, no. 4504, pp. 220–222, 1981.
[12] A. Malliani, M. Pagani, F. Lombardi, and S. Cerutti, “Cardio-
vascular neural regulation explored in the frequency domain,”
Circulation, vol. 84, no. 2, pp. 482–492, 1991.
[13] N. Montano, T. G. Ruscone, A. Porta, F. Lombardi, M. Pagani,
and A. Malliani, “Power spectrum analysis of heart rate vari-
ability to assess the changes in sympathovagal balance during
graded orthostatic tilt,” Circulation, vol. 90, no. 4 I, pp. 1826–
1831, 1994.
[14] S. H. Hyun, J. W. Im, W. S. Jung et al., “Effect of ST36
acupuncture on hyperventilation-induced CO2 reactivity of the
basilar and middle cerebral arteries and heart rate variability in
normal subjects,” Evidence-Based Complementary and Alterna-
tive Medicine, vol. 2014, Article ID 574986, 7 pages, 2014.
[15] W. Guangjun, T. Yuying, J. Shuyong, Z. Wenting, and Z.
Weibo, “Bilateral Hegu acupoints have the same effect on the
heart rate variability of the healthy subjects,” Evidence-Based
Complementary and Alternative Medicine, vol. 2014, Article ID
106940, 5 pages, 2014.
[16] Y. Tsunetsugu, B.-J. Park, H. Ishii, H. Hirano, T. Kagawa,
and Y. Miyazaki, “Physiological effects of Shinrin-yoku (taking
in the atmosphere of the forest) in an old-growth broadleaf
forest in Yamagata Prefecture, Japan,” Journal of Physiological
Anthropology, vol. 26, no. 2, pp. 135–142, 2007.
[17] B.-J. Park, Y. Tsunetsugu, H. Ishii et al., “Physiological effects of
Shinrin-yoku (taking in the atmosphere of the forest) in a mixed
forest in Shinano Town, Japan,” Scandinavian Journal of Forest
Research, vol. 23, no. 3, pp. 278–283, 2008.
[18] B.-J. Park, Y. Tsunetsugu, T. Kasetani, T. Morikawa, T. Kagawa,
and Y. Miyazaki, “Physiological effects of forest recreation in a
young conifer forest in Hinokage Town, Japan,” Silva Fennica,
vol. 43, no. 2, pp. 291–301, 2009.
[19] B. J. Park, Y. Tsunetsugu, T. Kasetani, T. Kagawa, and Y.
Miyazaki, “The physiological effects of Shinrin-yoku (taking in
the forest atmosphere or forest bathing): evidence from field
experiments in 24 forests across Japan,” Environmental Health
and Preventive Medicine, vol. 15, no. 1, pp. 18–26, 2010.
[20] C. Song, D. Joung, H. Ikei et al., “Physiological and psychologi-
cal effects of walking on young males in urban parks in winter,”
Journal of Physiological Anthropology, vol. 32, no. 1, article 18,
2013.
[21] C. Song, H. Ikei, M. Igarashi, M. Miwa, M. Takagaki, and Y.
Miyazaki, “Physiological and psychological responses of young
males during spring-time walks in urban parks,” Journal of
Physiological Anthropology, vol. 33, article 8, 2014.
[22] H. Ikei, C. Song, M. Igarashi, T. Namekawa, and Y. Miyazaki,
“Physiological and psychological relaxing effects of visual stim-
ulation with foliage plants in high school students,” Advances in
Horticultural Science, vol. 28, no. 2, pp. 111–116, 2014.
[23] M. Igarashi, M. Aga, H. Ikei, T. Namekawa, and Y. Miyazaki,
“Physiological and psychological effects on high school students
of viewing real and artificial pansies,” International Journal of
Environmental Research and Public Health, vol. 12, no. 3, pp.
2521–2531, 2015.
[24] Task Force of the European Society of Cardiology and the North
American Society of Pacing and Electrophysiology, “Heart rate
variability—standards of measurement, physiological interpre-
tation, and clinical use,” Circulation, vol. 93, no. 5, pp. 1043–1065,
1996.
[25] A. F. Bennett, “Interindividual variability: an under-utilized
resource,” in New Directions in Ecological Physiology, M. E.
Feder, A. F. Bennett, W. W. Burggren, and R. B. Huey, Eds., pp.
147–169, Cambridge University Press, Cambridge, UK, 1987.
[26] H. Kobayashi, B.-J. Park, and Y. Miyazaki, “Normative ref-
erences of heart rate variability and salivary alpha-amylase
in a healthy young male population,” Journal of Physiological
Anthropology, vol. 31, no. 1, article 9, 2012.
[27] H. Kobayashi, “Does paced breathing improve the repro-
ducibility of heart rate variability measurements?” Journal of
Physiological Anthropology, vol. 28, no. 5, pp. 225–230, 2009.
[28] V. F. Gladwell, D. K. Brown, J. L. Barton et al., “The effects
of views of nature on autonomic control,” European Journal of
Applied Physiology, vol. 112, no. 9, pp. 3379–3386, 2012.
[29] D. R. Gold, A. Litonjua, J. Schwartz et al., “Ambient pollution
and heart rate variability,” Circulation, vol. 101, no. 11, pp. 1267–
1273, 2000.
[30] C. A. Pope III, M. L. Hansen, R. W. Long et al., “Ambient par-
ticulate air pollution, heart rate variability, and blood markers
of inflammation in a panel of elderly subjects,” Environmental
Health Perspectives, vol. 112, no. 3, pp. 339–345, 2004.

Evidence-Based Complementary and Alternative Medicine
7
[31] Z. T. Fan, Q. Meng, C. Weisel et al., “Acute exposure to
elevated PM2.5 generated by traffic and cardiopulmonary health
effects in healthy older adults,” Journal of Exposure Science and
Environmental Epidemiology, vol. 19, no. 5, pp. 525–533, 2009.
[32] E. O. Wilson, Biophilia: The Human Bond with Other Species,
Harvard University Press, Cambridge, UK, 1984.
[33] E. Gullone, “The biophilia hypothesis and life in the 21st
century: increasing mental health or increasing pathology?”
Journal of Happiness Studies, vol. 1, no. 3, pp. 293–322, 2000.
[34] R. S. Ulrich, “Biophilia, biophobia, and natural landscapes,” in
The Biophilia Hypothesis, S. R. Kellert and E. O. Wilson, Eds.,
pp. 73–137, Island Press, Washington, DC, USA, 1993.
[35] M. Sarlo, D. Palomba, A. Angrilli, and L. Stegagno, “Blood
phobia and spider phobia: two specific phobias with different
autonomic cardiac modulations,” Biological Psychology, vol. 60,
no. 2-3, pp. 91–108, 2002.
[36] G. P. Prigatano and H. J. Johnson, “Autonomic nervous system
changes associated with a spider phobic reaction,” Journal of
Abnormal Psychology, vol. 83, no. 2, pp. 169–177, 1974.

MEDIATORS
of
INFLAMMATION
The Scientific
World Journal
Hindawi Publishing Corporation
http://www.hindawi.com
Volume 2014
Gastroenterology
Research and Practice
Hindawi Publishing Corporation
http://www.hindawi.com
Volume 2014
Hindawi Publishing Corporation
http://www.hindawi.com
Volume 2014
Journal of
Diabetes Research
Hindawi Publishing Corporation
http://www.hindawi.com
Volume 2014
Disease Markers
Hindawi Publishing Corporation
http://www.hindawi.com
Volume 2014
Journal of
Immunology Research
Hindawi Publishing Corporation
http://www.hindawi.com
Volume 2014
PPAR Research
Hindawi Publishing Corporation
http://www.hindawi.com
Volume 2014
Submit your manuscripts at
http://www.hindawi.com
Journal of
Obesity
International Journal of
Endocrinology
Hindawi Publishing Corporation
http://www.hindawi.com
Volume 2014
BioMed
Research International
Hindawi Publishing Corporation
http://www.hindawi.com
Volume 2014
Journal of
Ophthalmology
Hindawi Publishing Corporation
http://www.hindawi.com
Volume 2014
Stem Cells
International
Hindawi Publishing Corporation
http://www.hindawi.com
Volume 2014
Evidence-Based
Complementary and
Alternative Medicine
Hindawi Publishing Corporation
http://www.hindawi.com
Volume 2014
Hindawi Publishing Corporation
http://www.hindawi.com
Parkinson’s
Disease
Volume 2014
Journal of
Oncology
Hindawi Publishing Corporation
http://www.hindawi.com
Volume 2014
Computational and
Mathematical Methods
in Medicine
Hindawi Publishing Corporation
http://www.hindawi.com
Volume 2014
Behavioural
Neurology
Hindawi Publishing Corporation
http://www.hindawi.com
Volume 2014
Hindawi Publishing Corporation
http://www.hindawi.com
Volume 2014
AIDS
Research and Treatment
Hindawi Publishing Corporation
http://www.hindawi.com
Volume 2014
Oxidative Medicine and
Cellular Longevity
Hindawi Publishing Corporation
http://www.hindawi.com
Volume 2014
