
Int. J. Environ. Res. Public Health 2015, 12, 1874-1893; doi:10.3390/ijerph120201874
OPEN ACCESS
Article
International Journal of
Environmental Research and
Public Health
ISSN 1660-4601
www.mdpi.com/journal/ijerph
Acute Effects of Exposure to a Traditional Rural Environment
on Urban Dwellers: A Crossover Field Study in Terraced
Farmland
Juyoung Lee 1,†, Bum-Jin Park 2,†, Tatsuro Ohira 3,†, Takahide Kagawa 3,† and
Yoshifumi Miyazaki 4,*
1 Korea Forest Service, Government Complex 1, 189 Cheongsa-Ro, Seo-Gu, Daejeon 302-701,
Korea; E-Mail: lohawi@gmail.com
2 College of Agriculture and Life Sciences, Chungnam National University, 99 Daehak-Ro,
Yuseong-Gu, Daejeon 305-764, Korea; E-Mail: bjpark@cnu.ac.jp
3 Forestry and Forest Products Research Institute, 1 Matsunosato, Tsukuba 305-8687, Japan;
E-Mails: otatsu@ffpri.affrc.go.jp (T.O.); kagawa@ffpri.affrc.go.jp (T.K.)
4 Center for Environment, Health and Field Sciences, Chiba University, 6-2-1 Kashiwanoha,
Kashiwa, Chiba 277-0882, Japan
† These authors contributed equally to this work.
* Author to whom correspondence should be addressed; E-Mail: ymiyazaki@faculty.chiba-u.jp;
Tel.: +81-4-7137-8113; Fax: +81-20-4666-0398.
Academic Editor: Paul B. Tchounwou
Received: 25 December 2014 / Accepted: 30 January 2015 / Published: 5 February 2015
Abstract: Despite an increasing attention and public preference for rural amenities,
little evidence is available on the health benefits of a rural environment. In this study, we
identified physiological and psychological benefits of exposure to a rural environment using
multiparametric methods. Twelve young male adults participated in a 3-day field experiment
(mean ± standard deviation age, 22.3 ± 1.3 years). Sleeping environment, diet program,
physical activities, and other factors possibly affecting physiological responses were
controlled during experiment period. For all participants, salivary cortisol concentration,
heart rate variability, and blood pressure were measured at rural and urban field sites.
Self-evaluation questionnaires were administered to analyze the psychological states in two
different environments. Volatile compounds in the air were also analyzed to investigate air

Int. J. Environ. Res. Public Health 2015, 12
1875
quality. The data were compared between rural and urban environments. The data showed
that exposure to a rural environment reduced stress hormone secretion and sympathetic
nervous activity and increased parasympathetic nervous activity. Short-term exposure to a
rural environment also improved mood states. Our findings indicate that exposure to a rural
environment effectively reduced physiological stress and enhanced psychological well-being.
Keywords: terraced paddy field; physiological and psychological response; stress reduction;
health benefit of rural environment
1. Introduction
More than 50% of the world’s population currently lives in cities [1]. Urbanization is one of the
most fundamental characteristics in environmental changes, involving a broad range of environmental
issues such as landscape change [2], air pollution [3], and climate warming [4]. Urbanization has often
been regarded as a potential health risk factor in the field of environmental health [5]. To date,
an increasing number of studies have shown negative health effects of exposure to urban stimulations
in urban areas [6–8]. WHO (2010) [9] points out that urban environments tend to discourage physical
activity because of a variety of factors, including high-volume traffic, heavy use of motorized
transportation, and poor air quality. Recent studies have reported that urbanization is increasingly
linked with chronic non communicable diseases, including mental health disorders, obesity, type II
diabetes, metabolic syndrome, and cardiovascular disease [10–16], which is partly associated with
nutritional transition in modern society [17].
On the other hand, increasing attention has been given to the health benefits of exposure to natural
environments [18]. Since the late 20th century, a substantial body of research has illustrated the
positive effects of exposure to natural environments on varied psychological parameters, including
stress reduction, mood state promotion, recovery from fatigue, improved attention, and enhanced job
satisfaction [19–28]. Compared with physical activity in an urban setting, physical activity in a rural
setting is known to be more advantageous from the aspect of restoration [29,30]. Epidemiological
investigations have shown that contact with natural environments is positively associated with health
parameters, such as mental health [31], reduced health inequality [32], and longevity in urban
seniors [33]. In addition, recent physiological studies have provided strong evidence supporting direct
health benefits of exposure to forest environments by investigating the central nervous activity [34],
autonomic nervous activity [35–38], endocrine activity [34,36–38], and immune function [39,40].
Social needs for rural amenities are rapidly growing with rising living standards, added leisure, and
recreational activities, and there is an increasing interest in health promotion [41,42]. Health concerns
regarding city living [8,43] stress the importance of rural amenities from the perspective of health
promotion of urban dwellers. Rural amenities have become one of the most critical factors in the recent
trend of rural migration in US [44]. Recent studies have provided evidence supporting viewing rural
landscapes may provide positive health benefits [45]. One study reported that walking in a rural setting
was more advantageous to mood and mindset than walking in an urban setting [46].

Int. J. Environ. Res. Public Health 2015, 12
1876
Despite increasing attention and public preference for rural amenities [47,48], there is still
insufficient scientific evidence supporting the direct health benefits of rural environments. To address
this issue, measuring human physiological responses of subjects exposed to real environmental
stimuli would be the most valid method. This field approach has been applied in research on the
benefits of forests and has provided important evidence that could not be verified in indoor
experiments [34,39,49]. In addition, compared with an indoor approach, the field approach increases
the ability to generalize study effects [50]. Therefore, the aim of this study was to measure
physiological responses associated with exposure to a rural environment to investigate the potential
acute health benefits in urban dwellers.
2. Experimental Section
2.1. Subjects and Study Sites
The subjects were 12 young adult male students recruited from a local university. The mean age of
the participants was 22.3 ± 1.3 years (mean ± standard deviation). In the recruiting process,
the following exclusion criteria were used: past and current mental disorders, cardiovascular or allergic
diseases, and smoking or drinking habits. Before the study, the aims and protocol of the study were
concretely explained, and written informed consent was obtained from every participant. The names of
the participants were randomly coded. This study was conducted after obtaining approval from the
Ethics Committee of the Center for Environment, Health and Field Sciences, Chiba University.
To examine the physiological and psychological effects when exposed to real rural environments, a
traditional paddy field landscape in Ukiha City in southern Japan was selected as the study site
(Figure 1). The terraced paddy field is one of the typical rural landscapes in many Asian countries and
has high scenic value. As a control, an urban site around the Hakata station, which is one of the largest
railway terminals in southern Japan, was selected because the railway terminal is the most frequently
used facilities in Japan. The field study was conducted in autumn, and the weather was generally
pleasant throughout the study.
Figure 1. Rural landscape with terraced paddy field in Ukiha City (Left) and urban
landscape with traffic and buildings in Fukuoka City (Right) in southwestern Japan.

Int. J. Environ. Res. Public Health 2015, 12
1877
2.2. Experimental Design
To examine the acute effects of contact with traditional rural landscapes on urban dwellers, human
physiological and psychological responses at field sites were measured. Throughout the experiment,
the time schedule, meal and water intake, sleeping environment, and physical activity of the
participants were controlled to exclude variables, except for environmental stimuli, that may have
affected the subjects’ physiological conditions. The field experiment was conducted for 3 days, and all
participants stayed in the same type of single room in a hotel during the experimental period.
They were switched to a controlled schedule to control their physical activities. The participants’ meals
were provided according to a scheduled menu throughout the experiment. Breakfast, lunch, and dinner
were prepared to provide the equal nutrition and calories to each participant. Intake of caffeine,
including coffee, tea, and soft drinks, as well as smoking and drinking were prohibited. During the
daytime, all participants participated in the field experiment or remained in a waiting and read books.
After completing the field experiment, all participants stayed at a hotel and spent time watching
television or reading books. They were prohibited from going out at night, and the sleeping time was
between 10 PM and 6 AM. Other factors that could possibly influence the physiological or
psychological responses, such as hot spa bathing and use of cell phones and music players, were
also controlled.
On the first day of the experiment, all participants were gathered in a prepared room, and a general
explanation of this study was provided. Then, the participants previsited the field sites in the rural and
urban landscapes where the physiological and psychological measurements would be made so that the
participants could easily understand the experimental process. A previsit is important for reducing data
errors and clearly capturing the effects of environmental stimuli because it eliminates the
psychological tension caused by the first experience. On the second day, all participants were
randomly divided into two groups and allocated to rural or urban sites. The first set of physiological
and psychological data was obtained immediately after waking up at the hotel as a baseline (Table 1).
After breakfast, all participants traveled to each designated field site by car. Variations in the travel
time were minimized by adjusting the moving routes, irrespective of the study site. At each site,
measurements were made on one person at a time. Each participant rested in a seated position to
exclude the effects of physical activity and stabilize the physiological condition before measurements.
Then, the second set of data was measured during the pre-exposure period. According to the protocol,
each participant viewed the rural or urban landscape for 15 min, during which a participant was fully
exposed to real environmental stimuli, such as scene, sound, smell, and air quality. After exposure,
the third set of data was collected during the post-exposure period. For heart rate variability (HRV),
the data were recorded continuously throughout the exposure. On the third day, each participant was
assigned to another field site, and the data were obtained by following the same protocol as used on the
second day.

Int. J. Environ. Res. Public Health 2015, 12
1878
Table 1. Baseline values of the subjects in rural and urban environments.
Physiological parameters
Pulse rate(bpm)
SBP(mmHg)a
DBP(mmHg)a
ln(HF)
ln(LF/HF)
Psychological parameters
SD
Comfortable feeling
Soothed feeling
Natural feeling
Refreshed feeling
POMS
Tension-anxiety
Depression
Anger-hostility
Fatigue
Confusion
Vigor
Rural
Mean
SE
59.1
3.0
116.0
2.1
61.7
1.9
6.5
0.2
−2.3
0.7
2.5
0.5
1.9
0.7
−1.2
1.0
47.5
5.0
46.7
3.2
46.8
3.3
43.8
2.8
47.4
3.9
47.5
2.7
43.1
2.6
Urban
Mean
SE
61.5
3.6
122.2
3.5
64.1
2.0
6.1
0.4
−3.1
0.8
Differences
ns
ns
ns
ns
ns
1.5
0.5
ns
2.3
0.7
ns
−1.0
0.8
ns
52.7
4.1
ns
44.7
4.4
ns
47.2
3.0
ns
41.5
1.9
ns
46.4
4.2
ns
49.4
3.8
ns
40.9
2.6
ns
Notes: a SBP, systolic blood pressure; b DBP, diastolic blood pressure.
2.3. Measurement
2.3.1. Physiological Parameters
As indices of autonomic nervous activity, systolic blood pressure, diastolic blood pressure, and
pulse rate were measured using a portable blood pressure monitor (HEM-1000; Omron, Tokyo, Japan).
HRV, a parameter currently used to assess sympathetic and parasympathetic activities, was measured
using a portable electrocardiograph (Activtracer AC-301A; GMS, Tokyo, Japan). Autonomic functions
were investigated in all measurement periods at both the rural and urban sites. As an index of
endocrine activity, salivary cortisol, a reliable stress hormone that shows human stress reactions, was
investigated. Saliva samples were collected using a salivette (No. 51.1534; Sarstedt, Nümbrecht,
Germany), and the cortisol concentration was analyzed. The sampling method is very simple and
noninvasive. Saliva samples were taken before and after exposure to the environmental stimuli and the
values were compared. Saliva samples taken at the field sites were immediately placed in a freezer and
sent to a laboratory (SRL Inc., Tsukuba, Japan) for analysis of cortisol levels.
2.3.2. Questionnaires
Subjective evaluation methods were applied to measure the psychological responses to
environmental stimuli. The semantic differential method [51] was used to explore the participants’
perceptions on the two different environments. The semantic differential scale asks the subjects
to rate an impression of each environment on a 13-point scale that has two bipolar adjectives

Int. J. Environ. Res. Public Health 2015, 12
1879
(comfortable–uncomfortable, soothed–awakened, natural–artificial) at each end. The feeling of
refreshment was investigated using a questionnaire with 30 questions which had a total score range of
0–90 [52]. This questionnaire, a commonly used stress response checklist, contains multiple
adjectives that are rated by subjects on a 4-point scale to ascertain the degree to which they felt
refreshed. These psychological reactions were examined in all measurement periods. In addition,
the shortened Japanese version of the Profile of Mood States (POMS) [53] was used to assess the
following six mood dimensions on a 13-point scale: “tension–anxiety (T–A)”, “depression (D)”,
“anger–hostility (A–H)”, “confusion (C)”, “vigor (V)”, and “fatigue (F)”. The POMS tests, a widely
used psychological rating scale applied to assess transient mood states, were administered during the
pre- and post-exposure periods.
2.3.3. Air Quality Analysis
While considering the health effects of natural environments, less attention has been given to
volatile organic compounds (VOCs), which may have affected the health outcomes of urban dwellers.
The air samples were taken to analyze VOCs in the atmosphere of the two study sites. Rural samples
were taken near the terraced paddy field at an elevation of 400–450 m located in Ukiha City, and the
urban samples were taken in the Fukuoka City area in southern Japan. The organic constituents in the
air were trapped in glass cartridges (PEJ-02; Supelco, Bellefonte, PA, USA), which were filled with an
adsorbent (140 mg of Carboxen 1000 and 100 mg of Carbopac B, 60–80 mesh). The adsorbent tubes
were conditioned three times for 30 min at 295 °C in a helium gas flow of approximately 10 mL/min.
A total amount of 147 L of rural air was sampled for 24.5 h, and a total of 39 L of city air was sampled
for 6.5 h (sampling pump: MP-Σ30; Shibata, Tokyo, Japan) 1.2-m above the ground.
An ATD 400 automatic thermodosorption (PerkinElmer, Waltham, MA, USA) device coupled
with gas chromatography–mass spectrometry (GC–MS) was used for analysis. The trapped volatiles in
the adsorbent tube (PEJ-02) were preheated at 240 °C for desorption of the volatiles from the adsorbent
in a heater block with a heat controller for 15 min and collected into a cold trapping tube (Air-monitoring
tube; PerkinElmer) at −30 °C. Then, the volatiles were flushed into the gas chromatograph from a cold
trapping tube in a heater block with a heat controller at 300 °C for 15 min.
The components were identified by GC–MS analysis. Analytical runs were performed on a
Hewlett-Packard 5973/6890 GC–MS (Hewlett Packard, Wilmington, DE, USA) equipped with
selected ion monitoring (SIM) functions. The chromatographic conditions were: GC analytical column,
HP-5MS (30-m length, 0.25-mm i.d.); temperature program, 40 °C (15 min), 40 °C (at 4 °C/min),
180 °C (15 min), 180 °C (at 5 °C/min), 280 °C (15 min), 280 °C; carrier gas, helium at 1.2 mL/min.
Mass spectra were obtained at 70 eV, and peak identity was confirmed by comparison with standards.
Because the monoterpene concentrations in the atmosphere were too low to record mass spectra,
SIM was applied. The ions used by SIM were m/z 68, 93, and 136 because these are typical of
monoterpene mass spectra. The concentrations of monoterpenes in the samples were usually
determined from the peak heights of the SIM chromatogram at m/z 93 using a calibration curve
prepared from standard solutions.

Int. J. Environ. Res. Public Health 2015, 12
1880
2.4. Data Analysis
HRV data were assessed at various frequency bands using an HRV software tool (MemCalc/win;
GMS, Tokyo, Japan). In a continuously recorded data, interbeat (R–R) intervals were obtained for a 1-
min segment using the maximum entropy method. In this study, the two major spectral components of
HRV, the variances of the low-frequency (LF; 0.04–0.15 Hz) band and high-frequency (HF; 0.15–0.4
Hz) band, were calculated [54]. The HF data can be used as an index of parasympathetic nervous
activity, and the LF/HF ratio can be used as an index of sympathetic nervous activity. HRV values
were expressed as the natural logarithm (ln). In the POMS test, the T-score was used for the analysis of
the POMS test. Total mood disturbance (TMD) was calculated by summing the five negative mood
dimensions and subtracting the vigor score. One of the 12 participants retired in the middle of the
experiment, and a total of 11 samples were used for data analysis.
Comparisons between rural and urban data were performed for all parameters. For comparisons of
the physiological data, a paired t-test was applied for each data set. The Wilcoxon signed-rank test was
used to compare psychological data. Statistical analysis was performed by using Microsoft Excel
(Microsoft Inc. Redmond, WA, USA), and subjective data were processed using SPSS 21.0
(IBM-SPSS Inc, Chicago, IL, USA). The statistical differences were considered significant at p < 0.05.
All values were expressed as the mean ± standard error (SE).
3. Results
3.1. Physiological Parameters
Our data revealed different physiological effects of exposure to the rural and urban environments.
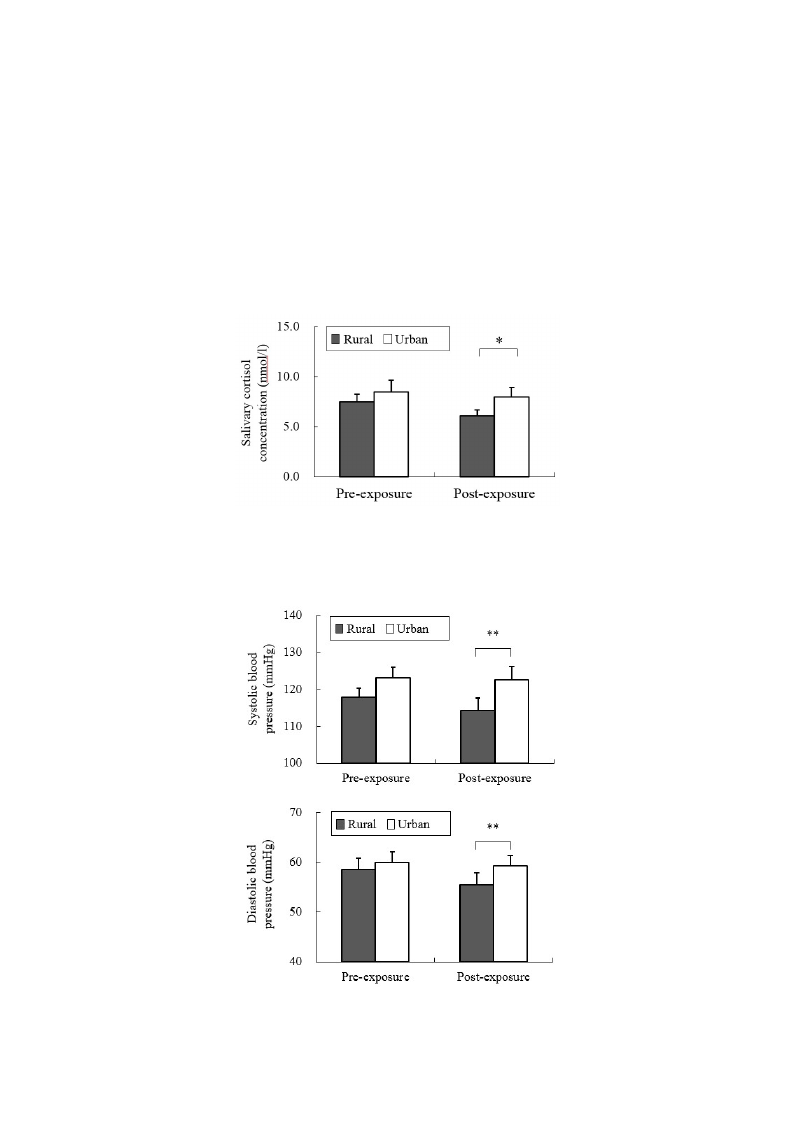
In the analysis of salivary cortisol concentration, a significant difference was found between the two
environments. When exposed to the rural environment, the level of salivary cortisol (6.07 ± 0.57 nmol/L)
was significantly decreased relative to that from urban exposure (7.95 ± 0.96 nmol/L; p < 0.05;
Figure 2), although no significant differences were observed in the pre-exposure period (rural,
7.47 ± 0.77 nmol/L; urban, 8.45 ± 1.17 nmol/L). Significant differences were identified for the
parameters of autonomic nervous activity. Systolic blood pressure after short-term exposure to real
environments was significantly decreased in the rural environment (114.1 ± 3.4 mmHg) relative to that
in the urban environment (122.6 ± 3.4 mmHg; p < 0.01; Figure 3 top), although no significant
differences were observed in the baseline (rural, 116.0 ± 2.1 mmHg; urban, 122.2 ± 3.5 mmHg) and
pre-exposure periods (rural, 117.8 ± 2.4 mmHg; urban, 123.0 ± 2.9 mmHg). Diastolic blood pressure
in the post-exposure period was significantly lower in the rural environment (55.4 ± 2.4 mmHg) than in
the urban environment (59.3 ± 2.1 mmHg; p < 0.01; Figure 3 middle), with no significant differences
in the baseline (rural, 61.7 ± 1.9 mmHg; urban, 64.1 ± 2.0 mmHg) and pre-exposure periods
(rural, 58.5 ± 2.2 mmHg; urban, 60.0 ± 2.1 mmHg). Pulse rate was significantly lower after exposure to
the rural (64.3 ± 2.2 beats/min), compared to the urban environments (67.5 ± 1.9 beats/min;
p < 0.05; Figure 3 bottom), although no significant differences were observed in the baseline
(rural, 59.1 ± 3.0 beats/min; urban, 61.5 ± 3.6 beats/min) and pre-exposure periods (rural,
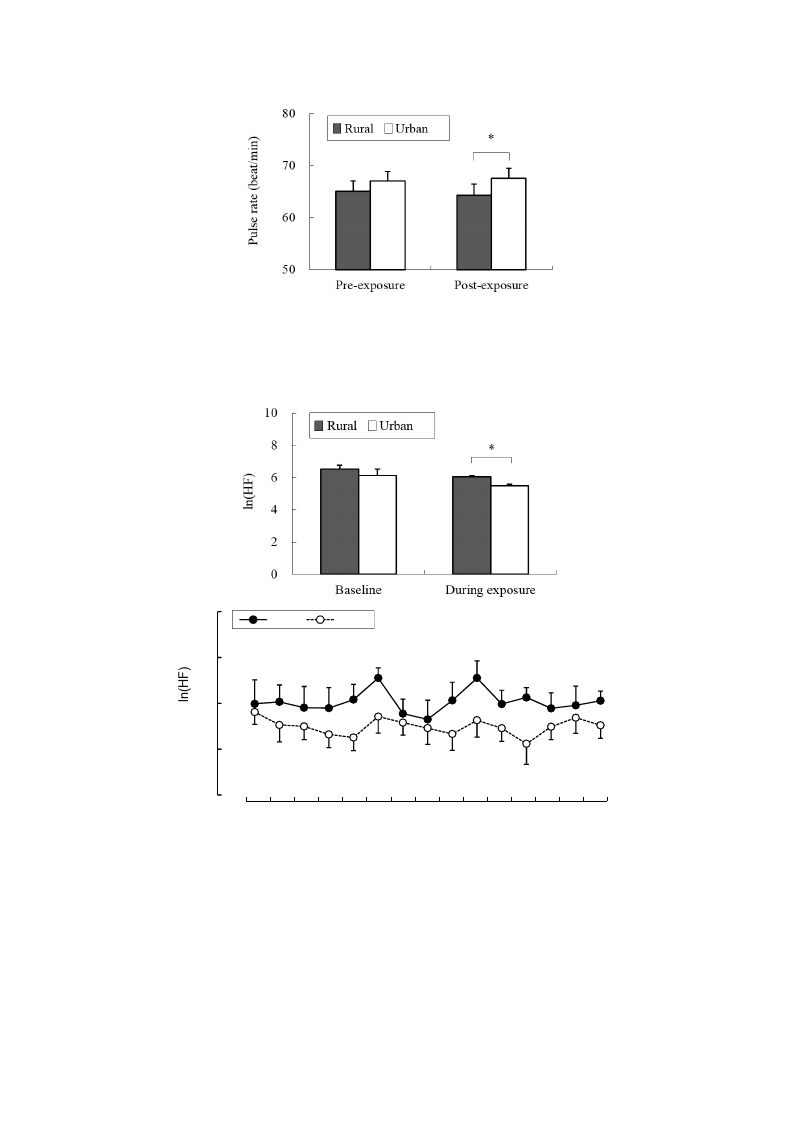
65.0 ± 2.0 beats/min; urban, 67.0 ± 1.9 beats/min) between the two environments. In the analysis of
HRV data, the mean 15-min ln(HF) values that reflected parasympathetic nervous activity were
Int. J. Environ. Res. Public Health 2015, 12
1881
significantly higher in the rural environment (6.03 ± 0.09) than in the urban environment (5.49 ± 0.08;
p < 0.01; Figure 4 top). The 1-min analysis of ln(HF) showed that the values were persistently higher
in the rural environment than in the urban environment (Figure 4 bottom). However, there were no
significant differences in the baseline values between the rural and urban environments. On the other
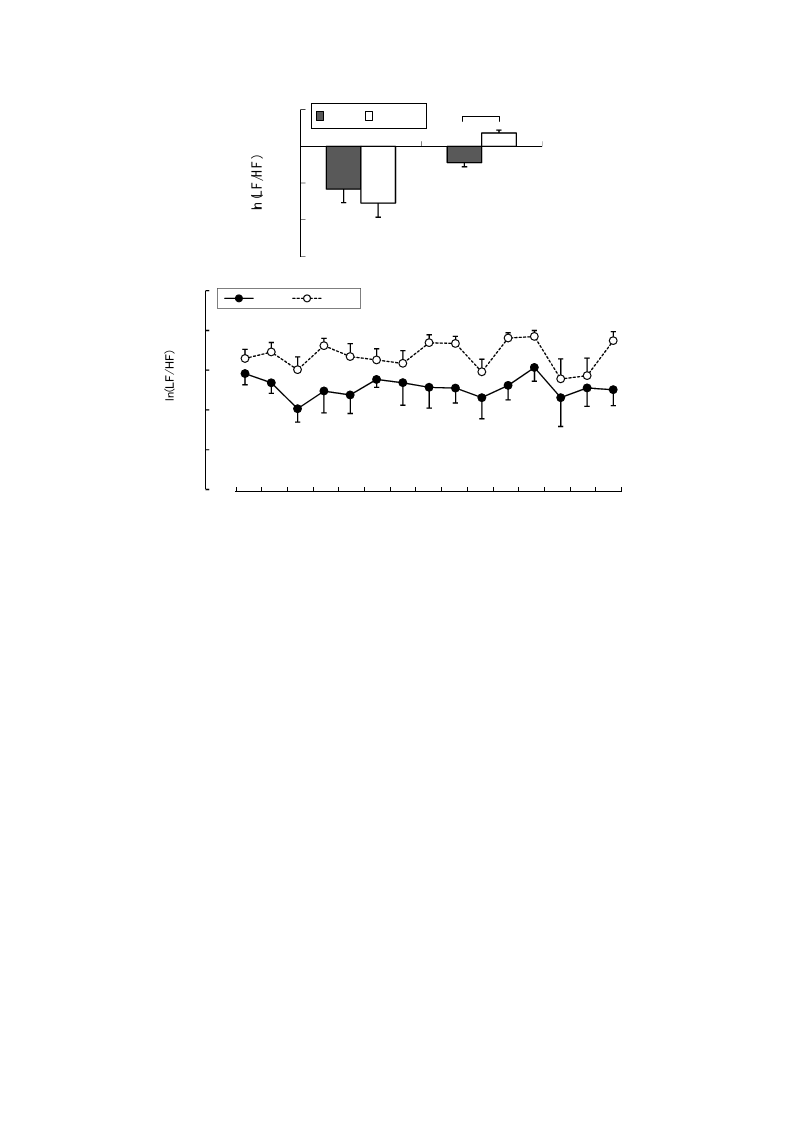
hand, the mean 15-min ln(LF/HF) values, which reflected sympathetic nervous activity, were
significantly lower in the rural environment (−0.89 ± 0.23) than in the urban environment (0.73 ± 0.15;
p < 0.01; Figure 5 top), although no significant differences were observed in the baseline values.
The 1-min analysis of ln(LF/HF) showed that the values were persistently lower in the rural
environment than in the urban environment during 15 min of exposure (Figure 5 bottom).
Figure 2. Comparison of salivary cortisol concentrations in participants at pre- and
post- exposure sessions between rural and urban environments. Mean ± SE; N = 11;
* p < 0.05; paired t-test.
Figure 3. Cont.
Int. J. Environ. Res. Public Health 2015, 12
1882
Figure 3. Comparison of systolic (top) and diastolic blood pressures (middle) and pulse
rate (bottom) between the rural and urban at pre- and post- exposure sessions. Mean ± SE;
N = 11; * p < 0.05; ** p < 0.01; paired t-test.
8
Rural
Urban
7
6
5
4
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15
Exposure time (min)
Figure 4. Comparison of the natural logarithm of the high frequency value in heart rate
variability between rural and urban exposures (top) and of the 1-min fluctuations of the
values (bottom) during exposure. Mean ± SE; N = 11; * p < 0.05; paired t-test.
Int. J. Environ. Res. Public Health 2015, 12
1883
2
Rural Urban
*
0
-2
-4
-6
B aseline
D uring exposure
4
Rural
Urban
2
0
-2
-4
-6
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15
Exposure time (min)
Figure 5. Comparison of the natural logarithm of high frequency/low frequency ratio in
heart rate variability between rural and urban exposures (top) and of 1-min fluctuations of
the value (bottom) during exposure. Mean ± SE; N = 11; * p < 0.05; paired t-test.
3.2. Psychological Parameters
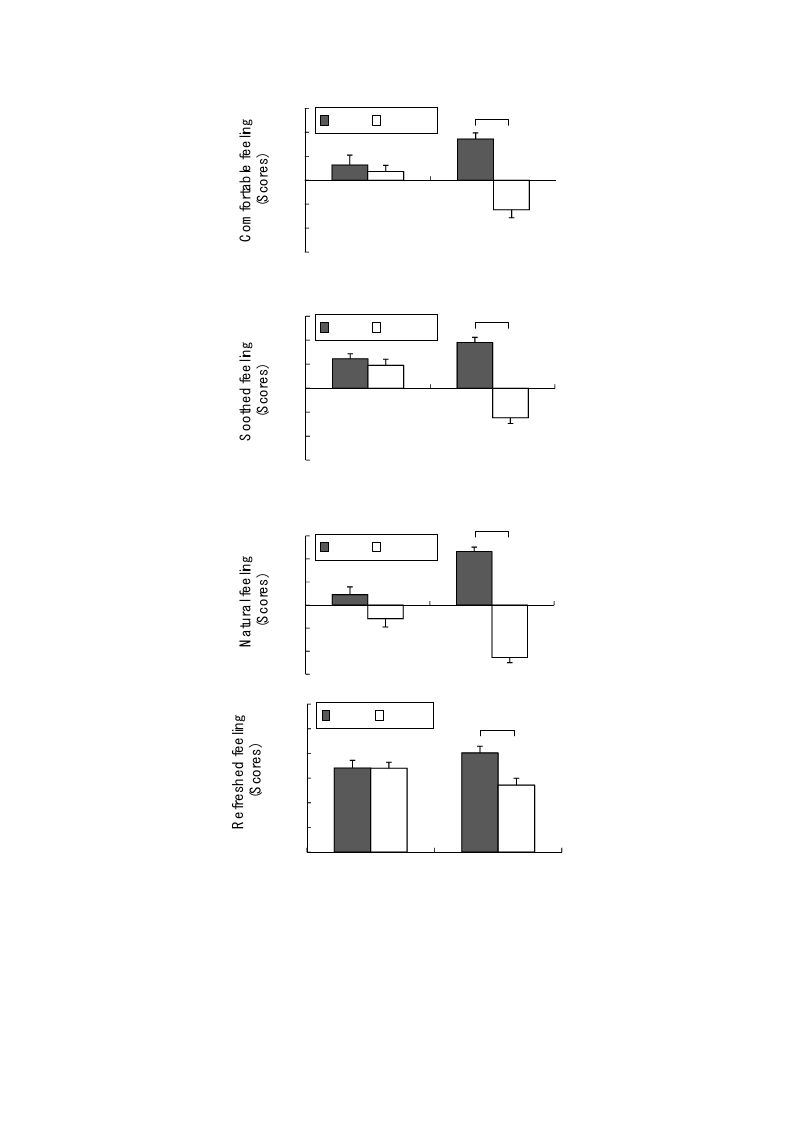
Compared with the urban environment, the rural environment had significantly positive effects on
the participants’ feeling and mood states. The participants responded that they felt significantly more
comfortable (rural, 3.5 ± 0.5; urban, −2.5 ± 0.7; p < 0.01; Figure 6 top), more soothed (rural, 3.8 ± 0.4;
urban, −2.5 ± 0.5; p < 0.01; Figure 6 middle top), more natural (rural, 4.6 ± 0.4; urban, −4.5 ± 0.5;
p < 0.01; Figure 6 middle bottom), and more refreshed (rural, 60.4 ± 4.1; urban, 40.7 ± 4.2; p < 0.01;
Figure 6 bottom) in the rural environment than in the urban environment, although no significant
differences were observed between the two in the baseline and pre-exposure periods. .In the POMS
analysis (Figure 7), significant differences were observed during the post-exposure period between the
rural and urban environments, respectively, for all of the subscale scores including those for T–A (42.7
± 2.3; 50.2 ± 3.8; p < 0.05), D (44.5 ± 2.8; 47.8 ± 4.0; p < 0.05), A–H (40.1 ± 1.7; 47.1 ± 4.4; p <
0.01), V (44.0 ± 2.3; 38.5 ± 2.4; p < 0.05), F (41.5 ± 2.6; 50.7 ± 4.0; p < 0.01), and C (45.6 ± 2.2; 50.9
± 3.9; p < 0.05). However, no significant differences were observed in the baseline period values
between the rural and urban environments, respectively: T–A (46.7 ± 3.2; 44.7 ± 4.4), D (46.8 ± 3.3;
47.2 ± 3.0), A–H (43.8 ± 2.8; 41.5 ± 1.9), V (43.1 ± 2.6; 40.9 ± 2.6), F (47.4 ± 3.9; 46.4 ± 4.2), and C
(47.5 ± 2.7; 49.4 ± 3.8).
Int. J. Environ. Res. Public Health 2015, 12
6
Rural Urban
**
4
2
0
-2
-4
-6
Pre-exposure
Post-exposure
1884
6
**
Rural Urban
4
2
0
-2
-4
-6
Pre-exposure
Post-exposure
**
6
Rural Urban
4
2
0
-2
-4
-6
Pre-exposure
Post-exposure
90
Rural Urban
75
**
60
45
30
15
0
Pre-exposure
Post-exposure
Figure 6. Comparison of the perceived comfortable (top), soothed (upper middle),
natural (lower middle), and refreshed feelings (bottom) between the rural and urban
environments at pre- and post- exposure sessions. Mean ± SE; N = 11; ** p < 0.01;
Wilcoxon signed-rank test.

Int. J. Environ. Res. Public Health 2015, 12
80
Rural Urban
60 * * ** ** * *
40
20
0
T-A
D
A-H
F
C
V
1885
Figure 7. Comparison of the Profile of Mood States (POMS) scores after exposure to the
rural and urban landscapes. Mean ± SE; N = 11; * p < 0.05; ** p < 0.01; Wilcoxon signed-
rank test. T–A, tension–anxiety; D, depression; A–H, anger–hostility; F, fatigue; C,
confusion; V, vigor.
3.3. Air Quality Analysis
There were considerable differences in the composition of volatile organic compounds between the
rural and urban air samples. In the rural samples, ambient VOCs of biogenic origins were abundant,
whereas in the urban samples, VOCs were mainly of anthropogenic origins with several contributions
from motor vehicles. Twelve terpenoids were identified in the rural air samples (Table 2A). The main
monoterpenes present in measurable amounts were α-pinene, camphene, D-limonene, and isoprene.
On the other hand, toluene and other aromatic compounds were dominant in the urban air samples,
with contributions from vehicle exhaust and industry processes (Table 2B). Urban air was also
characterized by various solvents including ethyl acetate, chloroform, dichloromethane, xylene,
1,2,4-trimethylbenzene, and n-hexane.
Table 2. (A) Terpenoids identified in rural air (Ukiha City, Japan) and (B) pollution
products and terpenoids identified in urban air (Fukuoka City, Japan). (A) Rural air
analysis; (B) Urban air analysis.
A
Compounds
Isoprene
Tricyclene
α-Pinene
Camphene
β-Pinene
Myrcene
δ-3-Carene
ρ-Cymene
D-Limonene
γ-Terpinene
α-Terpinolene
Bornyl acetate
Concentrations (ng/m3)
28.0
13.1
677.3
107.7
15.5
22.1
16.7
16.8
53.4
10.1
3.7
2.6
Int. J. Environ. Res. Public Health 2015, 12
These values are determined by absolute calibration
method using toluene.
Table 2. Cont.
B
Groups
Compounds
Concentrations (ng/m3)
n-Hexane
3414
2,4-Dimethylpentane
421
iso-Octane
450
Heptane
678
Octane
188
Alkanes
Nonane
459
Decane
622
Undecane
344
Dodecane
42
Tridecane
nd
Benzene
1481
Toluene
14,104
Ethylbenzene
1771
o, m, p-Xylene
2873
Styrene
139
Aromatic compounds
m-Ethyltoluene
p-Ethyltoluene
1024
424
1, 3, 5-Trimethylbenzene
412
o-Ethyltoluene
374
1, 2, 4-Trimethylbenzene
1870
1, 2, 3-Trimethylbenzene
337
1, 2, 4, 5-Tetramethylbenzene
35
α-Pinene
80
Terpenes
β-Pinene
5
D-Limonene
nd
Dichloromethane
5345
Chloroform
1686
1, 2-Dichloroethane
7
Halogenated compounds Trichloroethylene
16
1, 2-Dichloropropane
nd
Tetrachloroethylene
165
p-Dichlorobenzene
294
Esters
Ethyl acetate
Butyl acetate
5722
930
Nonanol
520
Alcohols
Decanol
125
Ethanol
nd
Acetone
nd
Aldehyde-ketone
Methylethylketone
nd
Methylisobutylketone
111
These values are determined by absolute calibration method using toluene.
nd: not detected.
1886

Int. J. Environ. Res. Public Health 2015, 12
1887
4. Discussion and Conclusions
The direct beneficial health effects of exposure to a rural environment relative to exposure to an
urban environment were evaluated in field experiments. This may be the pioneer study to show that
terraced paddy fields, a traditional agricultural landscape in many Asian countries, can be a health
promoter for modern urban dwellers.
Our data illustrates the physiological and psychological benefits of exposure to rural environments
more clearly than we expected. Evidence supported that exposure to rural environments can reduce
physiological stress by decreasing cortisol secretion which is associated with immune functions
mediated by the natural killer cell activity. Parameters reflecting autonomic nervous function showed
positive health benefits of exposure to rural environments, i.e., decreased pulse rate, blood pressure,
and sympathetic nervous activity (ln(LF/HF)) in addition to increased parasympathetic nervous activity
(ln(HF)). Exposure to a rural environment was also found to be effective for psychological relaxation
by increasing positive feelings and mood states and decreasing negative mood states.
To date, most of the studies that have explored the health-related effects of rural environments have
been conducted using indoor experiments and have investigated the limited effects of isolated
environmental stimulations under controlled indoor conditions [8]. Because health benefits are
obtained from the combination of all environmental stimuli, including views, sounds, smells, and air
quality, a field study can provide a better indication of the effects of real environments than can an
indoor study [50]. Although Roe and Aspinall [46] performed field experiments to investigate the
restoration effects of walking in a rural setting, their study had a limitation with respect to isolating the
effects solely from the rural environments because walking activity itself can affect health parameters.
In addition, the previous study did not suggest the physiological benefits of rural environments because
it used psychological parameters to investigate mood and cognitive characteristics. Therefore, the
present study is important because the field data clearly illustrated the health benefits of exposure to a
real rural environment and potential factors that could affect the participants’ physiological outcomes,
such as physical activity, diet-related conditions, and sleeping environments, were controlled.
Exposure to a real rural environment appears to be more beneficial than exposure to virtual rural
environments. Stress hormone secretion investigated by measuring salivary cortisol was found to
significantly decrease following rural exposure. The 1-min HRV analysis of HF and LF/HF showed
persistent differences in the values throughout the 15-min exposure period between rural and urban
environments. However, in a previous study conducted in a laboratory setting, the HRV effect was
observed only for 5 min of rural exposure [55]. Regarding the duration of the exposure effects, this
study indicated that exposure to real environmental stimuli can prolong the positive health effects
relative to those for exposure to laboratory stimuli. This finding may be associated with the overall
strength of the real stimuli provided by a combination of multiple environmental factors, such as
views, sounds, smells, and air quality, which may induce greater health benefits than those provided by
viewing isolated nature images in a laboratory. In the analysis of air quality, α-pinene was the most
abundant VOC in rural area. A previous indoor study by Tsunetsugu et al. [56] reported that Japanese
cedar scent, dominated by α-pinene compounds, can decrease systolic blood pressure and total
hemoglobin concentration in the prefrontal cortex. On the basis of this previous finding, we speculated
that the VOCs in the air in rural area might have affected the positive health outcomes in this study.

Int. J. Environ. Res. Public Health 2015, 12
1888
In addition, in laboratory research, the strength of the stimuli may affect the results because human
physiological and psychological responses can differ depending on how realistic the stimuli are, as
observed in a recent study that used two-dimensional and three-dimensional images to investigate the
prefrontal cortex and autonomic nervous activities [57].
The preference for natural environments has often been explained by the biophilia hypothesis [58],
attention recovery theory [22], and psycho-evolution theory [27]. These theories mainly approach this
issue from the perspective of psychology, and various psychological studies support the idea that a
natural environment is positively related to stress reduction, mood state promotion, recovery from
fatigue, and improved vitality [19–21,23,27,28,59]. However, these relationships cannot provide a
sufficient explanation for the health benefit mechanism. On the basis of increasing evidence on human
physiological reactions to nature in recent years, the preference for natural environments may be partly
explained by biological reactions to maintain homeostatic equilibrium [49]. Growing evidence from
experimental studies supports the idea that exposure to natural environments positively affects the
central nervous system [8,34,55], sympathetic and parasympathetic nervous systems [35,36,38,55],
endocrine system [34,36,37–39], and immune systems [39,40]. For example, decreased immune
function associated with chronic stress and fatigue recovered to normal levels following 3 days of
nature experience [39,40]. The nervous, endocrine, and immune systems are interrelated [60], which
also contributes to mental health conditions, such as anxiety and depression, through neurotransmitters
or hormones [61]. Therefore, this physiological evidence may help explain the fact that exposure to
natural environments is associated with positive health outcomes [32,33].
Urban environments in most developed countries have been planned and managed by mainly
focusing on the increase in convenience and efficiency without giving thorough consideration to the
effects of urban physical environments on human health. Most of the efforts regarding urban health
issues have been made to reduce the negative effects of urban pollution. In the recent years, with
increasing recognition of the fact that excess artificial environmental stimulation can cause negative
effects on individual and community health [7,62], more and more attention has been given to natural
environments. Our data suggest that visiting a rural environment may provide an effective chance for
stress reduction, particularly for urban dwellers at higher risk of stress-related health problems.
However, the health benefits identified in this study are not linked to the idea that rural dwellers are
healthier than urban dwellers because general health conditions are also related to many other factors,
including accessibility to health care service.
Despite the insufficient population size to generalize the present findings, they were
generally consistent with the findings of previous large-sample experiments performed in forest
environments [49,63,64]. Furthermore, given that being raised in a rural environment lowers the
prevalence of asthma and atopy among rural adolescents [65] and the risk of mental and physical
health problems in adulthood [66], exposure to rural environments needs to be considered as an
effective tool for management of modern health problems. A limitation of the study was that the
participants knew the purpose of the study, which was a potential source of bias and may have
influenced their answers to the psychological tests. Recommendations include further investigation of
the evidence in a larger population size with longer exposure and the mechanism underlying the health
benefits of rural environments. Close collaboration also should be undertaken among health

Int. J. Environ. Res. Public Health 2015, 12
1889
professionals, urban and rural planners, policy makers, and other concerned interest groups to utilize
exposure to rural environments as a new health promoting agent that may help reduce healthcare costs.
Acknowledgments
This project was supported by Grants-in-Aid for Scientific Research (S; 16107007) from the
Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Author Contributions
Juyoung Lee participated in the study design, carried out data collection and analysis, and drafted
the manuscript. Bum-Jin Park participated in the study design and carried out data collection and
analysis. Tatsuro Ohira participated in the collection and analysis of the VOCs. Takahide Kagawa and
Yoshifumi Miyazaki participated in the study design and data interpretation and edited the manuscript.
All authors read and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
1. Patel, R.B.; Burke, T.F. Urbanization—An emerging humanitarian disaster. N. Engl. J. Med.
2009, 361, 741–743, doi:10.1056/NEJMp0810878.
2. Antrop, M. Landscape change and the urbanization process in Europe. Landsc. Urban. Plan.
2004, 67, 9–26, doi:10.1016/S0169-2046(03)00026-4.
3. Mage, D.; Ozolins, G.; Peterson, P.; Webster, A.; Orthofer, R.; Vandeweerd, V.; Gwynne, M.
Urban air pollution in megacities of the world. Atmos. Environ. 1996, 30, 681–686,
doi:10.1016/1352-2310(95)00219-7.
4. Kalnay, E.; Cai, M. Impact of urbanization and land-use change on climate. Nature 2003, 423,
528–531, doi:10.1038/nature01952.
5. Jackson, R.J. The impact of the built environment on health: An emerging field. Amer. J. Public
Health 2003, 93, 1382–1384, doi:10.2105/AJPH.93.9.1382.
6. Frumkin, H. Beyond toxicity: Human health and the natural environment. Amer. J. Prev. Med.
2001, 20, 234–240, doi:10.1016/S0749-3797(00)00317-2.
7. IUHPE. The Evidence of Health Promotion Effectiveness: Shaping Public Health in a New
Europe; ECSC-EC-EAEC: Brussels, Belgium, 1999.
8. Lederbogen, F.; Kirsch, P.; Haddad, L.; Streit, F.; Tost, H.; Schuch, P.; Wüst, S.; Pruessner, J.C.;
Rietschel, M.; Deuschle, M.; Meyer-Lindenberg, A. City living and urban upbringing affect
neural social stress processing in humans. Nature 2011, 474, 498–501, doi:10.1038/nature10190.
9. WHO. Urbanization and health. Bull. WHO 2010, 88, 245–246, doi:10.2471/BLT.10.010410.
10. Adediran, O.S.; Adebayo, P.B.; Akintunde, A.A. Anthropometric differences among natives of
Abuja living in urban and rural communities: Correlations with other cardiovascular risk factors.
BMC Res. Notes 2013, 6, doi:10.1186/1756-0500-6-123.

Int. J. Environ. Res. Public Health 2015, 12
1890
11. Danaei, G.; Singh, G.M.; Paciorek, C.J.; Lin, J.K.; Cowan, M.J.; Finucane, M.M.; Farzadfar, F.;
Stevens, G.A.; Riley, L.M.; Lu, Y.; et al. Global burden of metabolic risk factors of chronic
diseases collaborating group: The global cardiovascular risk transition: Associations of four
metabolic risk factors with national income, urbanization, and Western diet in 1980 and 2008.
Circulation 2013, 127, 1493–1502.
12. Delisle, H.; Ntandou-Bouzitou, G.; Agueh, V.; Sodjinou, R.; Fayomi, B. Urbanisation, nutrition
transition and cardiometabolic risk: The Benin study. Brit. J. Nutr. 2012, 107, 1534–1544,
doi:10.1017/S0007114511004661.
13. Galea, S.; Uddin, M.; Koenen, K. The urban environment and mental disorders: Epigenetic links.
Epigenetics 2011, 6, 400–404, doi:10.4161/epi.6.4.14944.
14. Ginter, E.; Simko, V. Type 2 diabetes mellitus, pandemic in 21st century. Adv. Exp. Med. Biol.
2012, 771, 42–50.
15. Gong, P.; Liang, S.; Carlton, E.J.; Jiang, Q.; Wu, J.; Wang, L.; Remais, J.V. Urbanisation and
health in China. Lancet 2012, 379, 843–852, doi:10.1016/S0140-6736(11)61878-3.
16. Wagner, K.H.; Brath, H. A global view on the development of non communicable diseases.
Prev. Med. 2012, 54, S38–S41, doi:10.1016/j.ypmed.2011.11.012.
17. Logan, A.C.; Jacka, F.N. Nutritional psychiatry research: An emerging discipline and its
intersection with global urbanization, environmental challenges and the evolutionary mismatch.
J. Physiol. Anthropol. 2014, 33, 22, doi:10.1186/1880-6805-33-22.
18. Lee, J.; Li, Q.; Tyrväinen, L.; Tsunetsugu, Y.; Park, B.J.; Kagawa, T.; Miyazaki, Y. Nature therapy
and preventive medicine. In Public Health–Social and Behavioral Health; Maddock, J., Ed.;
Intech Online Publisher: Rijeka, Croatia, 2012; pp. 325–350.
19. Hartig, T.; Mang, M.; Evans, G.W. Restorative effects of natural environment experience.
Environ. Behav. 1991, 23, 3–26, doi:10.1177/0013916591231001.
20. Herzog, T.R.; Black, A.M.; Fountaine, K.A.; Knotts, D.J. Reflection and attentional recovery
as distinctive benefits of restorative environments. J. Environ. Psychol. 1997, 17, 165–170,
doi:10.1006/jevp.1997.0051.
21. Kaplan, R.; Kaplan, S. The Experience of Nature: A Psychological Perspective; Cambridge
University Press: Cambridge, UK, 1989.
22. Kaplan, S. The restorative benefits of nature: Toward an integrative framework. J. Environ.
Psychol. 1995, 15, 169–182, doi:10.1016/0272-4944(95)90001-2.
23. Korpela, K.M.; Yién, M. Perceived health is associated with visiting natural favourite places in
the vicinity. Health Place 2007, 13, 138–151, doi:10.1016/j.healthplace.2005.11.002.
24. Shin, W.S. The influence of forest view through a window on job satisfaction and job stress.
Scand. J. Forest Res. 2007, 22, 248–253, doi:10.1080/02827580701262733.
25. Taylor, F.A.; Kuo, F.E.; Sullivan, W.C. Coping with ADD: The surprising connection to green
play settings. Environ. Behav. 2001, 33, 54–77, doi:10.1177/00139160121972864.
26. Ulrich, R.S. View through a window may influence recovery from surgery. Science 1984, 224,
420–421, doi:10.1126/science.6143402.
27. Ulrich, R.S.; Simons, R.F.; Losito, B.D.; Fiorito, E.; Miles, M.A.; Zelson, M. Stress recovery
during exposure to natural and urban environments. J. Environ. Psychol. 1991, 11, 201–230,
doi:10.1016/S0272-4944(05)80184-7.

Int. J. Environ. Res. Public Health 2015, 12
1891
28. Verderber, S. Dimensions of person-window transactions in the hospital environments.
Environ. Behav. 1986, 18, 450–466, doi:10.1177/0013916586184002.
29. Hartig, T.; Evans, G.W.; Jamner, L.D.; Davis, D.S.; Gärling, T. Tracking restoration in natural and
urban field settings. J. Environ. Psychol. 2003, 23, 109–123, doi:10.1016/S0272-4944(02)00109-3.
30. Berman, M.G.; Jonides, J.; Kaplan, S. The cognitive benefits of interacting with nature.
Psychol. Sci. 2008, 19, 1207–1212, doi:10.1111/j.1467-9280.2008.02225.x.
31. Alcock, I.; White, W.P.; Wheeler, B.W.; Fleming, L.E.; Depledge, M.H. Longitudinal effects on
mental health of moving to greener and less green urban areas. Environ. Sci. Technol. 2013, 48,
1247–1255, doi:10.1021/es403688w.
32. Mitchell, R.; Popham, F. Effect of exposure to natural environment on health inequalities:
An observational population study. Lancet 2008, 372, 1655–1660, doi:10.1016/S0140-
6736(08)61689-X.
33. Takano, T.; Nakamura, K.; Watanabe, M. Urban residential environments and senior
citizens’ longevity in megacity areas: The importance of walkable green spaces. J. Epidemiol.
Community Health 2002, 56, 913–918, doi:10.1136/jech.56.12.913.
34. Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Hirano, H.; Kagawa, T.; Sato, M.; Miyazaki, Y.
Physiological effects of Shinrin-yoku (taking in the atmosphere of the forest)-using salivary
cortisol and cerebral activity as indicators. J. Physiol. Anthropol. 2007, 26, 123–128,
doi:10.2114/jpa2.26.123.
35. Lee, J.; Park, B.J.; Tsunetsugu, Y.; Kagawa, T.; Miyazaki, Y. Restorative effects of viewing real
forest landscapes: Based on a comparison with urban landscapes. Scand. J. Forest Res. 2009, 24,
227–234, doi:10.1080/02827580902903341.
36. Lee, J.; Park, B.J.; Tsunetsugu, Y.; Ohira, T.; Kagawa, T.; Miyazaki, Y. Effect of forest bathing
on physiological and psychological responses in young Japanese male subjects. Public Health
2011, 125, 93–100, doi:10.1016/j.puhe.2010.09.005.
37. Sung, J.; Woo, J.M.; Kim, W.; Lim, S.K.; Chung, E.J. The effect of cognitive behavior
therapy-based “Forest Therapy” program on blood pressure, salivary cortisol level, and quality of
life in elderly hypertensive patients. Clin. Exp. Hypertens. 2012, 34, 1–7, doi:10.3109/
10641963.2011.618195.
38. Tsunetsugu, Y.; Park, B.J.; Ishii, H.; Hirano, H.; Kagawa, T.; Miyazaki, Y. Physiological effects
of “Shinrin-yoku” (taking in the atmosphere of the forest) in an old-growth broadleaf forest in
Yamagata prefecture, Japan. J. Physiol. Anthropol. 2007, 26, 135–142, doi:10.2114/jpa2.26.135.
39. Li, Q.; Morimoto, K.; Nakadai, A.; Inagaki, H.; Katsumata, M.; Shimizu, T.; Hirata, Y.; Suzuki, H.;
Miyazaki, Y.; Kagawa, T.; et al. Forest bathing enhances human natural killer activity and
expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 2007, 20, 3–8.
40. Li, Q.; Morimoto, K.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.;
Shimizu, T.; Li, Y.J.; Wakayama, Y.; et al. A forest bathing trip increases human natural killer
activity and expression of anti-cancer proteins in female subjects. J. Biol. Regul. Homeost. Agents.
2008, 22, 45–55.
41. Rogge, E.; Nevens, F.; Gulinck, H. Perception of rural landscapes in Flanders: Looking beyond
aesthetics. Landsc. Urban Plan 2007, 82, 159–174, doi:10.1016/j.landurbplan.2007.02.006.

Int. J. Environ. Res. Public Health 2015, 12
1892
42. Fleischer, A.; Tsur, Y. The amenity value of agricultural landscape and rural-urban land
allocation. J. Agric. Econ. 2008, 60, 132–153, doi:10.1111/j.1477–9552.2008.00179.x.
43. Lopez, R. Urban sprawl and risk for being overweight or obese. Amer. J. Public Health 2004, 94,
1574–1579, doi:10.2105/AJPH.94.9.1574.
44. McGranahan, D.A. Landscape influence on recent rural migration in the U.S. Landsc. Urban Plan
2008, 85, 228–240, doi:10.1016/j.landurbplan.2007.12.001.
45. Kim, T.H.; Jeong, G.W.; Baek, H.S.; Kim, G.W.; Sundaram, T.; Kang, H.K.; Lee, S.W.;
Kim, H.J.; Song, J.K. Human brain activation in response to visual stimulation and rural urban
scenery pictures: A functional magnetic resonance imaging study. Sci. Total Environ. 2010, 408,
2600–2607, doi:10.1016/j.scitotenv.2010.02.025.
46. Roe, J.; Aspinall, P. The restorative benefits of walking in urban and rural settings in adults
with good and poor mental health. Health Place 2011, 17, 103–113, doi:10.1016/
j.healthplace.2010.09.003.
47. Sayadi, S.; González-Roa, M.C.; Calatrava-Requena, J. Public preferences for landscape features:
The case of agricultural landscape in mountainous Mediterranean areas. Land Use Policy 2009,
26, 334–344, doi:10.1016/j.landusepol.2008.04.003.
48. Harvey, T.; Works, M.A. Urban sprawl and rural landscape: Perceptions of landscape as amenity
in Portland, Oregon. Local Environ. 2002, 7, 381–396, doi:10.1080/1354983022000027509.
49. Lee, J.; Tsunetsugu, Y.; Takayama, N.; Park, B.J.; Li, Q.; Song, C.; Komatsu, M.; Ikei, H.;
Tyrväinen, L.; Kagawa, T.; et al. Influence of forest therapy on cardiovascular relaxation in young
adults. Evid-Based Compl. Alt. Med. 2014, 2014, doi:10.1155/2014/834360.
50. Groenewegen, P.G.; van den Berg, A.E.; de Vries, S.; Verheij, R.A. Vitamin G: Effects of green
space on health, well-being, and social safety. BMC Public Health 2006, 6, 149, doi:10.1186/
1471-2458-6-149.
51. Osgood, C.E.; Suci, G.J.; Tannenbaum, P.H. The Measurement of Meaning; University of Illinois
Press: Urbana, IL, USA, 1957.
52. Mackay, C.; Cox, T.; Burrows, G.; Lazzerini, T. An inventory for the measurement of
self-reported stress and arousal. Br. J. Soc. Clin. Psychol. 1978, 17, 283–284, doi:10.1111/j.2044-
8260.1978.tb00280.x.
53. Yokoyama, K.; Araki, S.; Kawakami, N.; Takeshita, T. Production of the Japanese edition of
Profile of Mood States (POMS): Assessment of reliability and validity. Jpn. J. Public Health
1990, 37, 913–918.
54. Task Force of the European Society of Cardiology; the North American Society of Pacing and
Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation
and clinical use. Circulation 1996, 93, 1043–1065, doi:10.1161/01.CIR.93.5.1043.
55. Brown, D.K.; Barton, J.L.; Gladwell, V.F. Viewing nature scenes positively affects recovery of
autonomic function following acute-mental stress. Environ. Sci. Technol. 2013, 47, 5562–5569,
doi:10.1021/es305019p.
56. Tsunetsugu, Y.; Park, B.J.; Miyazaki, Y. Physiological effects of visual, olfactory, auditory, and
tactile factors of forest environments. In Forest Medicine; Li, Q., Ed.; Nova Science Publisher:
Hauppauge, NY, USA, 2012; pp. 169–181.

Int. J. Environ. Res. Public Health 2015, 12
1893
57. Igarashi, M.; Yamamoto, T.; Lee, J.; Song, C.; Ikei, H.; Miyazaki, Y. Effects of stimulation by
three-dimensional natural images on prefrontal cortex and autonomic nerve activity: A comparison
with stimulation using two-dimensional images. Cogn. Process 2014, 15, 551–556,
doi:10.1007/s10339-014-0627-z.
58. Wilson, E.O. Biophilia: The Human Bond with Other Species; Harvard University Press:
Cambridge, MA, USA, 1984.
59. Takayama, N.; Korpela, K.; Lee, J.; Morikawa, T.; Tsunetsugu, Y.; Park, B.J.; Li, Q.;
Tyrväinen, L.; Miyazaki, Y.; Kagawa, T. Emotional, restorative and vitalizing effects of
forest and urban environments at four sites in Japan. Int. J. Environ. Res. Public Health 2014, 11,
7207–7230, doi:10.3390/ijerph110707207.
60. Scapagnini, U. Psychoneuroendocrinoimmunology: The basis for a novel therapeutic approach in
aging. Psychoneuroendocrinol 1992, 17, 411–420, doi:10.1016/0306-4530(92)90046-A.
61. Goodkin, K., Visser, A.P., Eds. Psychoneuroimmunology: Stress, Mental Disorders, and
Health;American Psychiatric Press: Washington, DC, USA, 2000.
62. Stilgoe, J.R. Gone barefoot lately? Amer. J. Prev. Med. 2001, 20, 243–244, doi:10.1016/S0749-
3797(00)00319-6.
63. Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The physiological effects of
Shinrin-yoku (taking in the forest atmosphere or forest bathing): Evidence from field experiments
in 24 forests across Japan. Environ. Health Prev. Med. 2010, 15, 18–26, doi:10.1007/s12199-009-
0086-9.
64. Tsunetsugu, Y.; Lee, Y.; Park, B.J.; Tyrväinen, L.; Kagawa, T.; Miyazaki, Y. Physiological and
psychological effects of viewing urban forest landscapes assessed by multiple measurements.
Landsc. Urban Plan 2013, 113, 90–93, doi:10.1016/j.landurbplan.2013.01.014.
65. Ernst, P.; Cormier, Y. Relative scarcity of asthma and atopy among rural adolescents raised on a
farm. Am. J. Respir. Crit. Care Med. 2000, 161, 1563–1566, doi:10.1164/ajrccm.161.5.9908119.
66. Goodwin, R.D.; Taha, F. Global health benefits of being raised in a rural setting: Results from
The National Comorbidity Survey. Psychiatr. Clin. Neurosci. 2014, 68, 395–403, doi:10.1111/
pcn.12144.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article
distributed under the terms and conditions of the Creative Commons Attribution license
(http://creativecommons.org/licenses/by/4.0/).
