
Nature´s Effect on Stress
in Women: A Systematic
Review
Bachelor Degree Project in Cognitive
Neuroscience
First Cycle 22.5 credits
Spring term Year 2022
Student: Sofia Rang
Supervisor: Monica Bergman
Examiner: Andreas Kalckert

2
Abstract
This systematic review aims to evaluate which effects nature exposure has on stress in
women and get a more objective viewpoint using measurements of physiological markers of
stress to complement the many studies using subjective questionnaires. A search was done on
Scopus, Medline EBSCO, and Web of Science for peer-reviewed, published, and original
research. Five studies met the inclusion criteria and were included in this review. The
outcome measurements included were activity in the autonomic nervous system (ANS)
measured with heart-rate variability (HRV) and cerebral activity measured by near-infrared
spectroscopy (NIRS). With the definition of Shinrin-Yoku in mind, nature exposure was
walking in or watching the natural environment, compared to walking in or watching an
urban environment. In this systematic review, four of five studies found significant results
that nature exposure alleviated stress in women compared to an urban environment. These
findings contribute to a growing body of evidence suggesting that nature is valuable in
reducing stress-related illnesses in women. On the individual level, these findings show that
nature exposure can be used as an evidence-based intervention to reduce stress in women.
Furthermore, these findings clarify the benefits of including elements from nature in urban
environments on a societal level.
Keywords: Nature, forest environment, stress, heart-rate variability, near-infrared
spectroscopy

3
Nature´s Effect on Stress in Women: A Systematic Review
Imagine the scent of a sun-kissed pine tree in the summer, the wind blowing in the
treetops making the sunlight flicker on the ground, and the sound of birds talking to each
other in the morning hour. Breath in the clean, fresh air and drop your shoulders. Why does
nature give so many of us a sense of calm and restore our energy? According to biophilia
(Wilson, 1984), later termed the biophilia hypothesis (Kellert & Wilson, 1993), humans have
lived on savannas for most of our evolution and therefore have a biological need to connect
with nature. Nature once helped us survive, which is why we feel comfortable there, so nature
is part of humans' evolutionary heritage (Gullone, 2000; Kellert & Wilson, 1993; Ulrich,
1993). For a considerably longer duration, the evolutionary adaptation of the brain took place
in savannah-like environments. Even though the brain still adapts, it is more adapted to
nature compared to today’s urban environment (Kellert & Wilson, 1993). During 99% of
human history, people have been hunter-gatherers. Thus, from an evolutionary perspective,
biophilic responses and landscape preferences may differ between biological factors such as
gender, age, and the presence of others. With gender differences regarding livelihood and
reproduction, men and women should evaluate and use the environment differently. Not
everyone will react equally to a specific environment because of a variance in vulnerability to
environmental threats and predation in the past (Kellert & Wilson, 1993).
Two theories focus on the relationship between the natural environment and mental
health: stress reduction theory (Ulrich et al., 1991) and attention restoration theory (Kaplan,
1995). According to Roberts et al. (2019) stress reduction theory proposes that the presence
of nature gives an evolutionary response of survival and safety, a response that generates
positive emotions. Attention restoration theory proposes that humans have a “soft
fascination” for the natural environment (Kaplan, 1995). This fascination allows people to
pay attention effortlessly to nature. Taking in the surroundings of nature without the
distractions of the urban environment provides precisely the amount of stimuli humans are
developed to handle (Dolling et al., 2017). Together, these theories indicate that people are
physiologically and psychologically less stressed when spending time in the types of nature
that have been important for our evolutionary history.
The growing separation between humans and nature has consequences for human
well-being (Hodson & Sander, 2017). Many things that follow urbanization are perceived as
dangerous to humans, often without consciously knowing it. Everyday things like
overcrowding, background sound, sudden loud noises, air pollution, and reckless drivers are
perceived as dangerous to our survival (Shuda et al., 2020). The growing urbanization makes
people more exposed to these stressors of the urban environment, which influence their
stress levels (Gruebner et al., 2017; Yao et al., 2021). An example of stressors in the urban
environment is that people need to constantly make quick decisions to move around in a
highly mobile and dense society. In addition, high-tech solutions demand our attention
(Dolling et al., 2017). A widespread and everyday phenomenon such as urban light exposure
can affect the circadian rhythm, changing people’s sleeping patterns, which alone can affect
their mental health (Gruebner et al., 2017). Thus, people who live in cities generally have a
higher risk for stress-related illnesses.
According to Gallup (2021) the feeling of experiencing high-stress levels worldwide
reached a record-high 40% in 2020. In 2019 that number was 35%. To get a clearer picture:
190 million more people worldwide experienced high-stress levels in just a year. The range in
reported stress from country to country was 13 to 66%, so not everyone felt stress to the same
degree. The incline began ten years ago, so therefore this development is not all due to the
pandemic (Gallup, 2021). According to Folkhälsomyndigheten (2022), 14% of the population
in Sweden between the age of 16-84 stated in 2020 that they feel stressed. When divided into
gender, 18% were women, and 11% were men. In other words, more women compared to men
felt stressed, and during 2006-2020, there was a slight increase in the population that was
stressed, and the increase in stress was highest in ages between 16–44. Stress-related
illnesses are increasing and have been since 1990. In the first quarter of 2020, mental illness

4
accounted for 41.3% of all ongoing illnesses. Women are more often on sick leave in contrast
to men, and more so due to mental illnesses. In general, women have a 25% higher risk of
getting ill, 31% for a mental illness, and for stress-related mental illness, that number is 41%
(Försäkringskassan, 2022).
For decades the default model for a human subject in research was a 70kg male, and
this model has provided ambiguous evidence about biology and health (Clayton, 2016). One
such example is the higher mortality rates for women with coronary heart disease due to the
deficient identification of symptoms for women (Dijkstra et al., 2008). Understanding
scientific findings in the framework of gender, differences, and similarities is essential for
applying research-based interventions that work for men and women (Clayton, 2016).
Considering this background within research and the fact that stress is a considerable larger
problem for women compared to men, it is of utmost importance to implement research in
this area on women.
Stress
The human body always strives for balance (homeostasis) and is designed with a
complicated range of metabolic systems to maintain normal balance (Thomas & Lena, 2010).
One definition of stress is that it is a response to a threatened balance of the body, which is
counteracted by a stress response that intends to re-establish the balance (Selye, 1956;
Thomas & Lena, 2010). Another definition is that stress is a maladaptive state caused by an
over-activated sympathetic nervous system (Campkin, 2000). Stress is the body's way of
reacting to a perceived environmental demand that can be valued as either threatening or
benign and is hard to avoid in everyday life (McEwen & Gianaros, 2010; Ridner, 2004;
Thomas & Lena, 2010). One stress response is the fight-and-flight response that has been
crucial for human survival. Fight-and-flight is designed to trigger body arousal seconds after
exposure to a stressor to stimulate a rapid reaction to get away safely (Klein & Corwin, 2002).
Although the fight-and-flight response is considered the traditional reaction for both men
and women to some stressors, women more often react with another response; tend-and-
befriend. Building and sustaining social relationships, tending and befriending promote
safety, reduce distress, and keeps the woman and potential offspring safe (Klein & Corwin,
2002).
Although the word stress is often negatively used, the underlying physiological
mechanism has been crucial to human survival throughout evolution. In the right amount,
stress can improve productivity (e.g., help us in a sudden threatening situation or help
students prepare for an exam) and, therefore, increase individual development. In contrast,
too much stress without breaks for restoration can be detrimental and cause chronic fatigue
and other psychological and physiological symptoms (Dolling et al., 2017; Hanoch & Vitouch,
2004). Stress-related illnesses increase, and people have less energy (Dolling et al., 2017).
According to Mulhall (1996), people must learn to either cope or be distressed with the
constraining forces of stress. According to Dolling et al. (2017) there are several symptoms of
stress (e.g., insomnia, increased heart rate, reductions in memory capacity, muscular aches,
and headaches) and stress-related physical symptoms (e.g., anxiety, nervousness, constant
fatigue, and severe pain in the neck and shoulders): symptoms that alone can affect people’s
work- or social life. Furthermore, if the stress develops into fatigue syndrome, it takes a long
time to recover, and after recovery, people generally stay more sensitive to stress.
The leading cause of stress among youth is school, life changes, relationship
problems, and wondering what career to pursue (Bhargava & Trivedi, 2018). For the adult
population, issues like family demands, work deadlines, job insecurity, or a long commute
may cause stress, among other things (Michie, 2002). Unpredictable and uncertain
situations, or situations involving conflicts or when life changes, are situations more likely to
cause stress (Michie, 2002; Mulhall, 1996). Although many sources may cause stress, this
review mainly focuses on increasing urbanization and how the natural environment can
affect stress.

5
The Stress Response
There are two major divisions within the human nervous system: the central and the
peripheral (Seo et al., 2010). The autonomic nervous system (ANS), part of the peripheral
nervous system, is associated with stress, among other negative states. ANS regulates the
automatic bodily functions associated with heart rate, digestion, breathing, and hormonal
systems (Seo et al., 2010). The sympathetic nervous system that initiates the stress response
and the parasympathetic nervous system that initiates the relaxation response are both parts
of ANS (Seo et al., 2010; Thomas & Lena, 2010). Thus, these two systems work together to
keep the body in homeostasis. When people are exposed to chronic stress, this balance can be
disturbed and cause stress-related health issues (Gazzaniga et al., 2013; Seo et al., 2010).
A whole cascade of events happens when the brain detects a threat to human
homeostasis intended to increase the probability of survival (Dijkstra et al., 2008; Seo et al.,
2010; Thomas & Lena, 2010). There can be either a psychological or physical stressor
threatening the body's balance, making the brain initiate a stress response, and a series of
chemical reactions follow. The stress response involves the release of hormones (e.g.,
norepinephrine and cortisol) and activation of the regulatory centers of the central nervous
system (amygdala, hippocampus, and prefrontal cortex). The amygdala and hippocampus
process experiences together with the brainstem, hypothalamus, and prefrontal cortex (PFC).
Moreover, whether an event is interpreted as stressful or not is based on present or past
experiences (McEwen & Gianaros, 2010). The amygdala, located in the medial anterior
temporal lobes, processes and activates emotions and behavior. The hippocampus, located in
the medial temporal lobe, determines the event's context and processes declarative and
episodic memory about the event (McEwen & Gianaros, 2010). If an event is interpreted as
stressful, these areas excite the hypothalamic-pituitary-adrenal (HPA) axis and ANS
(McEwen & Gianaros, 2010; Mello et al., 2003; Seo et al., 2010; Thomas & Lena, 2010). The
amygdala acts excitatory, and the hippocampus is in general inhibitory, although some areas
act excitatory. Medial PFC, located in the anterior frontal lobes, is involved in different higher
cognitive functions, one being the top-down regulation of stress. This regulation is mediated
by subcortical areas (amygdala, hippocampus, and hypothalamus), and numerous prefrontal
areas send direct projections to areas concerning the regulation of the stress response
(McEwen & Gianaros, 2010). The HPA axis interacts closely with the locus coeruleus-
norepinephrine system activating the fight-and-flight response. These systems are involved
in a substantial reciprocal innervation throughout the central nervous system to turn the
stress-response on and off (Dijkstra et al., 2008; McEwen & Gianaros, 2010; Mello et al.,
2003). This neural circuitry can be adaptive in the short term but maladaptive in the long
term (McEwen & Gianaros, 2010).
Methods to Measure Stress
Since stress depends on complex networks, measuring stress by a single marker is
impossible (Yao et al., 2021). Studies have used physiological parameters such as blood
pressure, pulse rate, heart rate variability (HRV), and salivary cortisol (Bedini et al., 2017;
Hjortskov et al., 2004; Largo-Wight et al., 2016). These physiological markers are indicatives
of central-autonomic activity or indicators of change in the immune and endocrine systems
(Seo et al., 2010). In addition, electroencephalogram (EEG) and near-infrared spectroscopy
(NIRS) are used to measure brain activity related to stress (Choi et al., 2015; Nagasawa et al.,
2020). Several studies have used subjective questionnaires to measure the psychological
aspects of stress (Balconi et al., 2019; Crivelli et al., 2019; Largo-Wight et al., 2016; Takayama
et al., 2019). This review will focus on methods that measure physiological markers, such as
EEG, NIRS, HRV, and salivary cortisol.
EEG measures the brain's electrical activity at the top of the scalp. The electrical
activity is measured by different bands of frequency, called waves. From high to low, these
bands are called: Delta, Alpha, Beta, and Gamma (Choi et al., 2015). The bands indicate

6
different functions of the brain and nervous system activity. High-beta waves in the temporal
lobe indicate a stress reaction, and alpha waves in the frontal lobe indicate a relaxed state
(Choi et al., 2015; Seo et al., 2010; Ulrich, 1981). Hence, this method can be indicative of
stress reactions.
NIRS is a method that measures brain activation by monitoring brain oxygenation
(Tsunetsugu & Miyazaki, 2005). NIRS is non-invasive, and the portable device makes it
suitable for field experiments (Tsunetsugu & Miyazaki, 2005). A decrease in brain
oxygenation in the prefrontal cortex indicates that cerebral activity has attenuated, indicating
a relaxed state (Park et al., 2007).
HRV is a measure of heart rate on a beat-to-beat basis, and the time interval can have
a variation of 10-30%, although the heart rate per minute remains constant (Kobayashi et al.,
1999). R-wave, the most prominent waveform of the electrocardiogram, is counted in
numbers: a parameter known as the R-R interval variation. The power spectrum of the HRV
signal is divided into frequency sections. A high-frequency component (HF, 0.15Hz-0.4Hz)
represents parasympathetic nerve activity related to relaxation. On the contrary, a low-
frequency component (LF, 0.04Hz-0.15Hz) represents sympathetic and parasympathetic
nerve activity, and the proportion of LF/(HF+LF) represents activity in the sympathetic
nervous system related to stress (Lim et al., 2021). HRV contributes to knowledge about
stress levels based on the autonomic nerve reactions and the underlying processes mediating
beat-to-beat changes (Kobayashi et al., 1999; Lim et al., 2021; Porges, 2007).
Cortisol is “a non-invasive indirect window on the brain” (Clow & Smyth, 2020, p. 2).
The concentration of cortisol circulating through the body changes from hour to hour, and
everyday emotions and thoughts can cause fluctuations in cortisol concentration. Adverse
events such as stress cause a spike in cortisol, while more enjoyable events cause a reduction
(Clow & Smyth, 2020). The differences in cortisol levels can be measured accurately by
measurements in the saliva (Clow & Smyth, 2020).
Nature as an Intervention
Shinrin-Yoku, also called forest bathing, is when one walks in a forest environment,
breathing in its air and watching it closely. In Japan, Shinrin-Yoku is a traditional practice
thought of as meditation or an artform (Antonelli et al., 2019; Park et al., 2010). A relatively
recent systematic review (Kondo et al., 2018) found evidence that spending time outdoors in
a preferably green environment may reduce the experience of stress. Other reviews indicate
that Shinrin-Yoku is useful as an intervention to reduce stress (Hansen et al., 2017; Oh et al.,
2017). The interest in Shinrin-Yoku in science originates from Japan but has spread to other
parts of the world (e.g., China, South Korea, Germany, Iceland, Finland, and Spain; Antonelli
et al., 2019).
World Health Organization (2022) recommends self-care interventions as a critical
path for every country to promote health and serve the vulnerable. Self-care interventions are
quality tools that are evidence-based and support individuals in managing their health care
without help from a healthcare worker (World Health Organization, 2022).
The Aim
This systematic review aims to investigate if there is evidence that nature affects
stress in women. By focusing on studies that measure participants' physiological responses
while spending time in nature, this thesis aims to get a more objective viewpoint of the field
as a complement to the many studies using subjective questionnaires. This review aimed to
include all four measurements (EEG, NIRS, HRV, and salivary cortisol). However, no studies
fulfilling the inclusion criteria used EEG or salivary cortisol. Hence, even though these
measurements are valuable in this field of research, this review could not include them. With
the definition of Shinrin-Yoku in mind and World Health Organizations recommendation for
self-care interventions, studies where participants walk, or take in the natural environment

7
on their own, without the help of a guide or a therapist, will be included. Like in other
disciplines, many studies are done with all-male participants (Kobayashi et al., 2018, 2019;
Lee et al., 2011; Mao et al., 2012). According to the biophilia hypothesis, there may be gender
differences in the way women and men respond to the natural environment (Kellert &
Wilson, 1993). Moreover, the all-male studies can provide ambiguous evidence on
interventions aimed at women (Clayton, 2016). With the increase of stress in women, it is
essential to find evidence-based interventions suited for them. Given the tend-and-befriend
response to stress, walking alone in nature might not work the same way for women as for
men. Therefore, this review will focus on all-female studies to gather evidence for future self-
care interventions in nature for women experiencing stress. By focusing on all-female studies,
this review will also balance the research area regarding gender. Stress is a growing problem,
as seen in the increasing number of people taking sick leave due to stress-related issues.
Finding affordable (nature is free) and evidence-based, easy-to-use interventions for people
to pursue on their own is crucial to relieving stress and decreasing stress-related illnesses in
our society.
Search Strategy
Methods
Regarding getting an overview of the subject, the initial search consisted of different
combinations of keywords (e.g., forest bathing, shinrin-yoku, forest therapy, natural
environment, forest exposure, forest walking and EEG, HRV, heart rate variability, cortisol).
After a closer look at several studies for more keywords and by reading about the different
measures used in stress-related research combined with nature, the final search string used
was ("forest environment" OR "restorative environment" OR "shinrin-yoku" OR "shinrin
yoku" OR "forest therapy" OR "forest bathing" OR "therapeutic effect of forest" OR "forest
environment" OR "forest landscape" OR "forest walking" OR "forest exposure") AND (nirs
OR "near-infrared spectroscopy" OR "near infrared spectroscopy" OR hrv OR "heart rate
variability" OR cortisol OR "salivary cortisol" OR "salivary cortisol concentration" OR eeg).
The search was set on title, abstract, and keywords for Scopus, and the quotation marks were
changed into curly brackets as informed by the library at the University of Skövde. There
were no restrictions set for Medline EBSCO or Web of Science, and the original search string
as seen above was used. The end date for the search was the 10th of March, 2022. The search
gave 285 articles on three different databases (Medline EBSCO n=50, Scopus n=93, Web of
Science n=142). The records of the articles were extracted, saved, and imported to Rayyan
(Ouzzani et al., 2016). First, 115 duplicates were removed. The remaining 170 articles were
screened by the title and abstract, and 136 additional studies were excluded due to not
meeting the inclusion criteria (see inclusion and exclusion criteria). The final step was to
screen the full text of the remaining 34 articles. The final screening excluded 29 articles due
to different population (n = 19), wrong study design (n = 6), foreign language (n = 2), wrong
outcome (n = 1), and no access (n = 1). The total sum of 5 studies was included in this
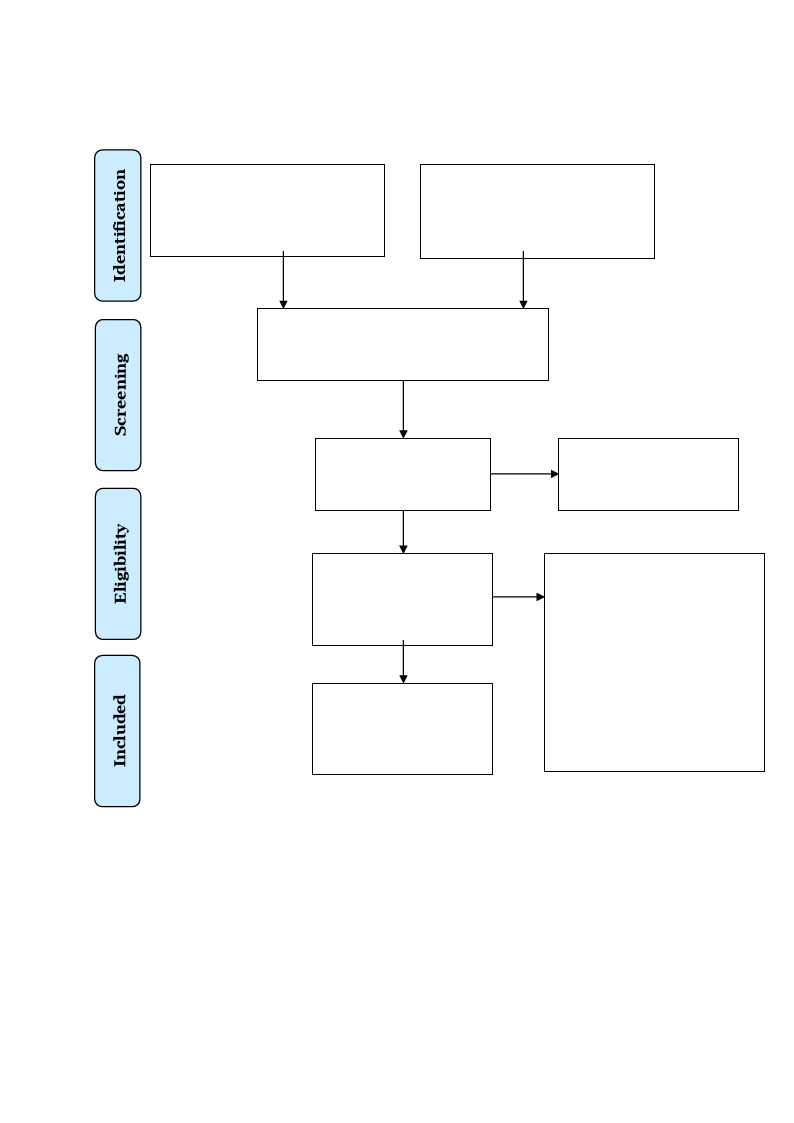
systematic review (see Figure 1).
Inclusion & Exclusion Criteria
The inclusion criteria for the participants are only adult (18+) female subjects within
all ethnicities and from all countries. Hence, all-male, mixed, and animal studies are
excluded. Participants can be with or without stress-related issues/illnesses. The inclusion
criteria for the intervention are studies with a nature-based intervention (e.g., forest bathing,
forest walk, or watching a natural environment) without any additional intervention or help
from a guide/therapist. Light exercise, like walking, in all time durations is allowed.
Consequently, studies with exercise programs, a joining therapist or guide, pictures of nature,
or virtual reality are excluded. The primary goal is to find studies that measure the
physiological outcome related to stress with the neuroimaging method of near-infrared
spectroscopy (NIRS) or electroencephalogram (EEG), but indirect methods of salivary
cortisol and HRV will also be included. Papers must be in English, peer-reviewed, published,

8
and be original research. Only studies available through the university database or open
access are included.
Data Extraction
The data extracted and presented from the included articles are as follows (eight
categories): the first author with the publication date, study design, and location, sample size,
age and study population, intervention, duration of intervention, control group, outcome
measurement, and results (see Table 1). Outcome measurements extracted are cerebral
activity measured by NIRS and activity in the autonomic nervous system measured with
HRV.
9
Figure 1
PRISMA 2009 Flow Diagram
Records identified through
database searching
(n = 285)
Additional records identified
through other sources
(n = 0)
Records after duplicates removed
(n = 170)
Records screened
(n = 170)
Records excluded
(n = 136)
Full-text articles
assessed for eligibility
(n = 34)
Studies included in
qualitative synthesis
(n = 5)
Full-text articles excluded,
with reasons
(n = 29)
Different population (n = 19)
Wrong study design (n = 6)
Foreign language (n = 2)
Wrong outcome (n = 1)
No access (n = 1)
Note: The literature search process, illustrated in a PRISMA 2009 Flow Diagram. Adapted
from Moher et al. (2009).

10
Results
This review identified five papers that met the inclusion criteria (see Table 1).
Although this review aimed to find and include studies using one or more of the following
methods: EEG, NIRS, HRV, or salivary cortisol, there were only studies using the methods
HRV and NIRS that fulfilled the inclusion criteria.
Study Characteristics
Across the five studies, there were 272 participants in total, and the data analysis are
based on the measurements from 214 participants. Sample sizes ranged from 17–72, with a
mean age of 21–46.1 in four studies. In the fifth study, the age ranged between 20–36 (see
Table 1). The study population ranged from young female university students to adult females
living in urban areas, healthy and free of psychological or physical disorders. Four of the five
studies (Igarashi et al., 2015; Song et al., 2019a, 2019b, 2020) were conducted in Japan, and
one study (Stigsdotter et al., 2017) was conducted in Denmark. All studies used a cross-
over/within-subject design where all the participants were exposed to each environment.
Four of the studies (Igarashi et al., 2015; Song et al., 2019a, 2019b, 2020) collected data
during the environmental exposure, while one study (Stigsdotter et al., 2017) collected data
before and after the environmental exposure.
The studies conducted two of four environmental exposures (see Table 1): watching
nature versus an urban environment or walking in nature versus walking in an urban
environment. Igarashi et al. (2015) used a kiwifruit orchard landscape as a natural
environment and a building site as an urban environment. The participants viewed the
kiwifruit orchard from the edge while sitting down, and if they turned around, they could see
the building site. However, when watching the building site, participants were seated in the
shadow of a tent closer to the buildings. The kiwifruit orchard consisted of 14 trees bearing
many fruits, and the leaves kept the sun away from the participants’ eyes. The building site
consisted of a two-story building and a well-paved road. In two of the studies by Song et al.
(2019a, 2019b) 12 different locations were used: six forest areas and six city areas. The
participants were divided into groups of twelve and assigned to one of each type of location.
In the third study by Song et al. (2020) ten different locations were used: five forest areas and
five city areas. The forest areas were well-maintained and safe, including trees such as oak,
red pine, maple, and cherry. The city areas were located either near a railway station or
downtown. Stigsdotter et al. (2017) used the Danish Health Forest, Octavia, an Arboretum
containing the most extensive collection of shrubs and trees in Denmark for the natural
environment. The walk consisted of a 750 m long trail exposed to open areas, a lake, more
secluded green areas, and a pine forest. For the city environment, the walk took place in an
area filled with architectonical and historical qualities in downtown Copenhagen. The city
area was chosen for its historical value in maybe not being as stressful as other urban areas.
The participants were transported in a minibus to both environments. Moreover, which
environment the participants would visit was told on the bus. The duration of the bus ride
was the same for both environments.
Watching nature as well as watching the urban environment involved a passive
exposure while sitting down at each site. When walking, participants did so along a given
course at an average pace of about 1km at each environmental site. The amount of time of the
environmental exposure across all the studies varied from 10 to 15 minutes.
Stress Measurements
This review identified two different outcome measurements of stress: activity in the
ANS measured with HRV (Holter, 1961) and cerebral activity measured by NIRS (Jöbsis,
1977). Each measurement involved one or more outcomes which are shown in Table 1.

11
Autonomic Nervous System
Four studies measured HRV as an indicator of ANS activity (Igarashi et al., 2015;
Song et al., 2019a, 2019b; Stigsdotter et al., 2017). All four studies monitored HRV using a
portable electrocardiograph. HRV was measured for its total power (TP), low frequency (LF)
component, high frequency (HF) component, and low frequency/high frequency (LF/HF)
ratio. Three of four studies (Igarashi et al., 2015; Song et al., 2019a, 2019b) found statistically
significant differences in the participants’ physiological responses between the natural and
urban environment.
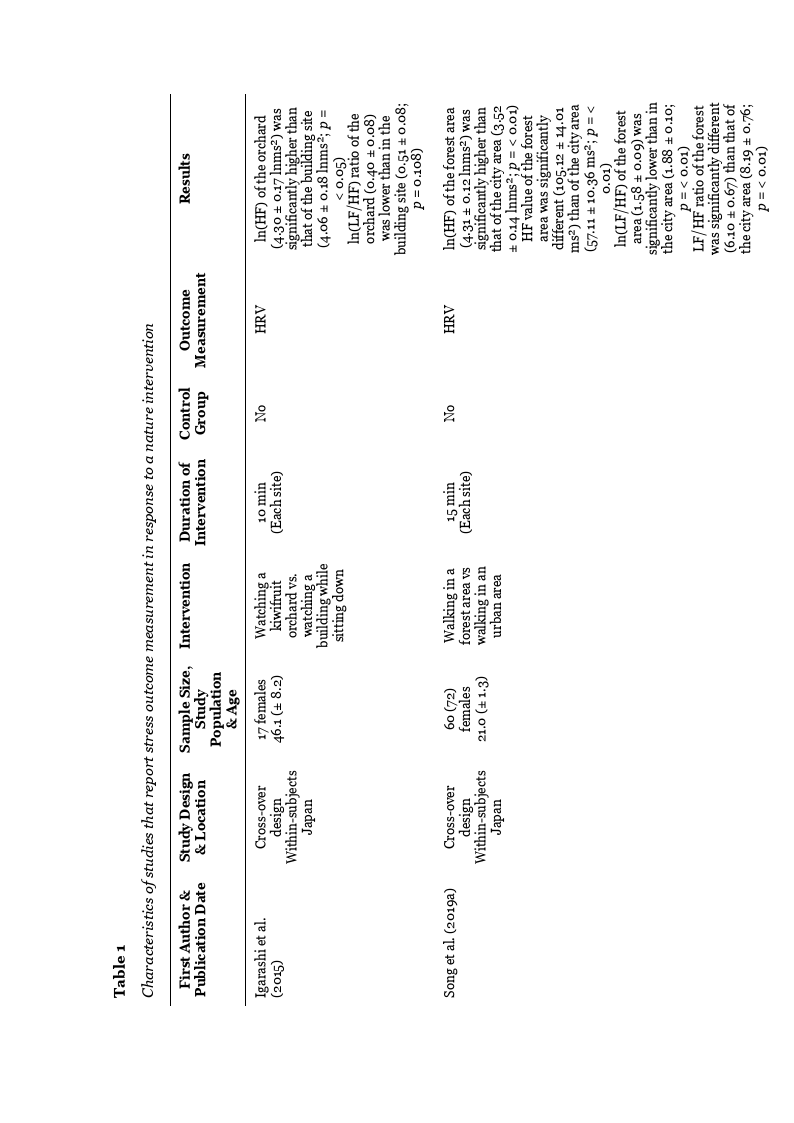
In the study by Igarashi et al. (2015) the mean ln(HF) value, an indicator of
parasympathetic nerve activity, was significantly higher for watching the kiwifruit orchard
compared to the building site. The ln(LF/HF) ratio, an indicator of sympathetic nerve
activity, was lower in the kiwifruit orchard in contrast to the building site. However, the
difference was not statistically significant (see Table 1).
In the study by Song et al. (2019a) the mean value of ln(HF) was significantly higher
when walking in nature compared to the urban environment. The non-logarithmic HF value
also showed a significant difference between the two environments, with the natural
environment being higher in contrast to the urban one. These results indicate a
parasympathetic activity. The ln(LF/HF) ratio was significantly lower for walking in nature
compared to the urban environment. The non-logarithmic LF/HF ratio value also showed a
significant difference indicating a reduced sympathetic nerve activity (see Table 1).
When the participants were sitting down instead of walking (Song et al., 2019b), they
showed a significant difference in the mean value of ln(HF) between watching nature and the
urban environment. When watching the natural environment, the ln(HF) was significantly
higher in contrast to watching the urban one. The non-logarithmic HF values also showed a
significant difference between the two environments. As in their earlier study (Song et al.,
2019a), the ln(LF/HF) ratio was also significantly lower for the natural environment in
comparison to the urban one. The same goes for the non-logarithmic LF/HF ratio (see Table
1).
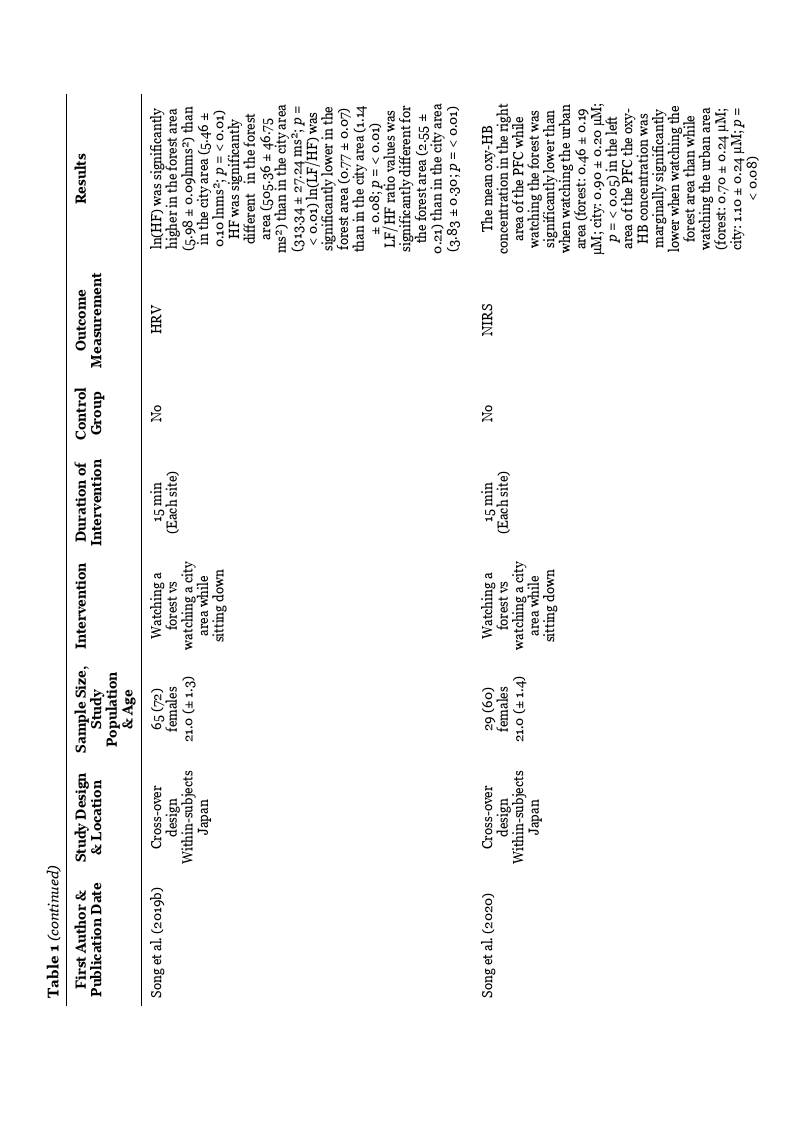
In the study by Stigsdotter et al. (2017) that used HRV as an indicator of ANS, no
statistically significant differences were shown between the mean value of ln(TP), ln(LF),
ln(HF), and ln(LF/HF) ratio value when comparing the natural environments to the urban
environment. However, comparing HRV measurements from the bus ride to measurements
taken before and after the intervention, a significant difference shows a more prominent
parasympathetic activity before and after the intervention, compared with the bus ride,
indicated by the mean values of ln(HF). Furthermore, the ln(LF/HF) ratio was significantly
lower before and after the intervention compared with the bus ride, and there was no
significant difference between before and after the intervention.
Cerebral Activity
One study used NIRS to measure cerebral activity (Song et al., 2020). A portable two-
channel NIRS device was used to measure the shifts in oxyhaemoglobin (oxy-HB)
concentration. The NIRS probes are flexible, and two sensors were placed on the participants’
foreheads over the right and left frontal regions.
The results of Song et al. (2020) show that the mean oxy-HB concentration was lower
in the right area of PFC when watching the natural environment compared to watching the
urban environment. The mean oxy-HB concentration in the left area of PFC was marginally
significantly lower when watching the natural environment compared to the urban
environment (see Table 1).
12
13

14

15
Discussion
This systematic review aimed to investigate if there is evidence that nature affects
stress in women. By focusing on studies that measure participants' physiological responses
while spending time in nature, this thesis aimed to get a more objective viewpoint of the field
as a complement to the many studies using subjective questionnaires. This review found
significant results in four of the studies that nature exposure alleviated physiological markers
of stress. However, the fifth study deviated from the rest and showed no significant difference
between the natural and urban environments.
The results of Igarashi et al. (2015) indicate an activation of the parasympathetic
nervous system and a suppression of the sympathetic nervous system in the kiwifruit orchard
compared to the building site. These results are in accordance with previous studies on men
(Lee et al., 2011; Park et al., 2008, 2009, 2010) where participants watched a forest area and
an urban area while sitting down.
As presented earlier, the results in the two studies by Song et al. (2019a, 2019b)
indicate an activation of the parasympathetic nervous system and a suppression of the
sympathetic nervous system. Similar results are found in previous studies that explored the
physiological responses to a forest environment in male participants. In three studies, the
participants viewed the forest in a seated position (Lee et al., 2011; Park et al., 2008;
Tsunetsugu et al., 2013), and in one study, the participants were walking in the forest (Lee et
al., 2014) and in three studies the participants were viewing from a seated position as well as
walking in the forest (Park et al., 2009, 2010). Even though the participants were walking in
Song et al. (2019a) and sitting down in Song et al. (2019b), the results were similar. However,
according to Song et al. (2019b) the proportion of participants showing these physiological
responses was higher for those walking in the forest compared to those sitting down and
watching the forest environment.
In the most recent study by Song et al. (2020) where they used NIRS to measure the
physiological responses to stress, the activity in the PFC was significantly decreased when
viewing the forest environment compared to the urban environment. Similar results were
found in previous studies where participants viewed a forest environment from a rooftop
(Joung et al., 2015) and where participants viewed a forest while sitting down as well as
walking in a forest (Park et al., 2007). A short walk in a forest environment decreased the
total hemoglobin concentration in the left area of PFC (Park et al., 2007). In another all-
female study (Song et al., 2018) where they used an image of a forest on a TV screen, there
was a reduction in oxy-HB concentrations in the right area of PFC. The difference between
these studies is that in the study by Park et al. (2007) the participants were walking in a
forest, while in Song et al. (2018) they were seated and watching a TV screen, and in Song et
al. (2020), the participants were seated and watched a natural forest environment.
Conditions with no physical activity resulted in a shift in activity in the right area compared
to the left area of PFC. Oxy-HB is affected by changes in skin blood flow from physical
activity (Miyazawa et al., 2013). However, no physical activity was part of the experiment
since it was performed in a seated position. Future research is needed for additional
information on the mechanisms associated with the right and left areas of PFC to get a clearer
picture of the differences between the two areas. Also, measuring skin blood flow
simultaneously with oxy-HB concentration can further establish if physical activity influences
the result.
All four studies so far have results that are linked to physiological relaxation.
Activation of the parasympathetic nervous system and suppression of the sympathetic
nervous system indicate a relaxed state (Kobayashi et al., 1999; Lim et al., 2021; Porges,
2007). Moreover, a decrease in brain oxygenation in the prefrontal cortex indicates that
cerebral activity has attenuated, indicating a relaxed state (Park et al., 2007).
On the contrary, Stigsdotter et al. (2017) did not get a significant difference between
the forest and the urban environment. Stigsdotter et al. (2017) did not measure HRV during

16
the intervention itself as the others did. Instead, the measurements were done before, after,
and during the bus ride to the two different locations. There was a significant difference
between the bus ride compared to the forest environment as well as compared to the city
environment. Thus, both environments were more physiologically restorative than being on
the bus. On the other hand, there was no significant difference between the forest and the
urban environment before or after walking in either environment. These findings contradict
previous studies (Joung et al., 2015; Lee et al., 2011, 2014; Park et al., 2007, 2008, 2009,
2010; Tsunetsugu et al., 2013) and Stigsdotter et al. (2017) mention that the type of urban
environment may explain the result. The choice to have an urban environment with historical
and architectural values and streets with very little traffic differ from the previously
mentioned studies. Previous studies have used most modern urban environments with more
traffic or modern houses: according to Staats et al. (2016), the least desired urban
environment for restoration. Furthermore, not measuring HRV during the intervention
makes it harder to compare these results with the other included studies in this review.
Two measurements were not included in this review: EEG and cortisol. Most studies
using EEG in nature/stress research use virtual reality or a forest therapy program. One
study (Hassan et al., 2018) fulfilled all the inclusion criteria except the participants were both
men and women. Hassan et al. (2018) used EEG to measure participants walking in either a
bamboo forest or an urban environment. The results indicate that the participants were
relaxed in the forest but under stress in the urban environment. Another study (Yu et al.,
2016) using salivary cortisol fulfilled all criteria except that a therapy program with other
elements than walking or observing nature was used. Nevertheless, the results indicate that
the participants were more relaxed two and four weeks after the forest therapy program.
There seem to be no gender differences in the way women and men respond to the
natural environment when comparing results from this review and earlier mentioned studies.
However, according to Song et al. (2019b) men get more relaxed when watching a forest from
within compared to walking in one. In contrast, women get more relaxed by walking in the
forest compared to watching the forest from a seated position (Song et al., 2019b). With
gender differences regarding livelihood and reproduction, men might evaluate being on the
lookout as more relaxing, and women who have more responsibility for their offspring might
react more relaxing when walking and searching for suitable shelter. Given the tend-and-
befriend response to stress, there may be more decisive differences if the same interventions
were done with a group of women. Hence, there needs to be more research on tend-and-
befriend response in this area of research. Moreover, most group interventions have the help
of a guide. Given the recommendation from World Health Organization to find self-care
interventions that people can pursue on their own, future research should focus on designing
suitable interventions for groups without the help of a guide.
Ethical and Societal Aspects
All five studies were approved by an ethics committee, and the participants signed a
written informed consent in advance. None of the studies declare any conflict of interest or
ethical dilemmas. There were no risks for the participants in the forest or urban
environments or using the different methods in the five studies. The participants were all
healthy, and none had any psychiatric or physiological disorders.
Stress is a significant problem in today's urban society, which is shown in the
increasing number of stress-related illnesses causing people to go on sick leave. If nature can
be a part of the solution to diminish stress and stress-related illnesses, this line of research is
valuable to our society. Nature is free and available in some form to many people. Hence, the
social status of people is irrelevant, making nature interventions available to almost
everyone.

17
Limitations
There are several limitations in the included five studies. In the studies by Song et al.
(2019a, 2019b, 2020) the participants were healthy young female university students,
making it difficult to generalize the results. Song et al. (2019a, 2019b) only measured
autonomic nervous activity as a physiological marker, which according to Yao et al. (2021)
does not capture the entire stress response. Using a mean value from numerous
environments makes it difficult to know if one environment is more effective in alleviating
stress compared to others (Song et al., 2019a, 2019b, 2020). Igarashi et al. (2015) and
Stigsdotter et al. (2017) used specific environments, and Stigsdotter et al. (2017) used a small
number of participants, making it difficult to generalize the results. Also, collecting data over
two seasons: spring and autumn may have led to bias due to vegetation differences in the two
seasons (Stigsdotter et al., 2017). Not having a corresponding measurement in all studies
makes it difficult to compare results within the field. Recruiting participants through posters
and notice boards, possibly only having nature-interested people sign up for the studies, can
also bias the results.
One of the most significant limitations of this review is the number of studies
included. The fact that the same researchers were involved in four of five studies is an overall
limitation. The number of participants in the included studies is also relatively low (three had
fewer than 60 participants). Furthermore, only having one study using NIRS limited the
possibility of comparing these results. Finally, this review has multiple selection biases:
including direct exposure only, not addressing laboratory environments of nature like VR or
pictures, only choosing open access articles or articles accessed via the school library, and
only including articles published in the English language.
Future Research
Future research should include more participants ranging from all age groups to be
more representative of the population. Furthermore, the tend-and-befriend reaction to stress
should be researched using group settings with single participant control groups to compare
the results. Future research is also needed to separate the different environments and find
the best-suited ones for future interventions. Further, regarding Stigsdotter et al. (2017)
there also needs to be research to explore the different urban environments.
More research is also needed using physiological markers of stress out in the natural
environment and not measured in a laboratory using virtual reality. Although virtual reality
may help relieve stress, being out in the natural environment adds to the importance of
caring for the natural environment for future generations. Additionally, testing out different
lengths of interventions and comparing these against each other, and doing follow-up testing
to see how long-lasting the effect of nature is on the participants, is another aspect to
consider for future research.
According to Yao et al. (2021) stress is impossible to measure using only a single
marker due to its complex networks. This review found only five studies using physiological
markers to measure stress in women, and none of them used EEG or salivary cortisol, and
very few exist at all that use EEG together with other methods out in natural forest
environments. With this lack of different physiological measurements, future research needs
to broaden its use of physiological measurements. Additionally, the framework needs to be
more cohesive throughout the field when using these measurements.
Conclusion
This review showed that nature alleviates stress in women, similar to previous
research on men, and adds to the existing knowledge of the effect of nature exposure on
women's stress. Hence, supporting the biophilia hypothesis, stress reduction theory, and

18
attention restoration theory. On a societal level, physicians and policymakers should be
aware of the importance of this knowledge. Physicians when planning for prevention or
treatment for many of the illnesses that follow from stress and policymakers when planning
new city landscapes and the importance of access to natural environments. Stress-related
illnesses could be a less frequent cause of sick leave in women if more elements from nature
were included in urban environments. On an individual level, the present review contributes
to the growing body of evidence suggesting that nature exposure is an evidence-based
intervention effective in alleviating stress in women.
19
References
Antonelli, M., Barbieri, G., & Donelli, D. (2019). Effects of forest bathing (shinrin-yoku) on
levels of cortisol as a stress biomarker: A systematic review and meta-analysis.
International Journal of Biometeorology, 63(8), 1117–1134.
https://doi.org/10.1007/s00484-019-01717-x
Balconi, M., Fronda, G., & Crivelli, D. (2019). Effects of technology-mediated mindfulness
practice on stress: Psychophysiological and self-report measures. Stress, 22(2), 200–
209. https://doi.org/10.1080/10253890.2018.1531845
Bedini, S., Braun, F., Weibel, L., Aussedat, M., Pereira, B., & Dutheil, F. (2017). Stress and
salivary cortisol in emergency medical dispatchers: A randomized shifts control trial.
PloS One, 12(5), e0177094. https://doi.org/10.1371/journal.pone.0177094
Bhargava, D., & Trivedi, H. (2018). A study of causes of stress and stress management among
youth. IRA-International Journal of Management & Social Sciences, 11(03), 108–117.
http://doi.org/10.21013/jmss.v11.n3.p1
Campkin, M. (2000). Stress management in primary care. Family Practice, 17(1), 98–99.
https://doi.org/10.1093/fampra/17.1.98--a
Choi, Y., Kim, M., & Chun, C. (2015). Measurement of occupants’ stress based on
electroencephalograms (EEG) in twelve combined environments. Building and
Environment, 88, 65–72. https://doi.org/10.1016/j.buildenv.2014.10.003
Clayton, J. A. (2016). Studying both sexes: A guiding principle for biomedicine. The Faseb
Journal, 30, 519–524. https://doi.org/10.1096/fj.15-279554
Clow, A., & Smyth, N. (2020). Salivary cortisol as a non-invasive window on the brain.
International Review of Neurobiology, 150, 1–16.
https://doi.org/10.1016/bs.irn.2019.12.003
20
Crivelli, D., Fronda, G., Venturella, I., & Balconi, M. (2019). Stress and neurocognitive
efficiency in managerial contexts: A study on technology-mediated mindfulness
practice. International Journal of Workplace Health Management, 12(2), 42–56.
http://doi.org/10.1108/IJWHM-07-2018-0095
Dijkstra, A. F., Verdonk, P., & Lagro-Janssen, A. L. M. (2008). Gender bias in medical
textbooks: Examples from coronary heart disease, depression, alcohol abuse and
pharmacology. Medical Education, 42, 1021–1028. https://doi.org/10.1111/j.1365-
2923.2008.03150.x
Dolling, A., Nilsson, H., & Lundell, Y. (2017). Stress recovery in forest or handicraft
environments – An intervention study. Urban Forestry & Urban Greening, 27, 162–
172. https://doi.org/10.1016/j.ufug.2017.07.006
Folkhälsomyndigheten. (2022, February). Stress.
https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/tolkad-
rapportering/folkhalsans-utveckling/resultat/halsa/stress/
Försäkringskassan. (2022, February). Stressrelaterad psykisk ohälsa: 41 procent högre risk
för kvinnor. https://www.forsakringskassan.se/nyhetsarkiv/nyheter-press/2020-09-
08-stressrelaterad-psykisk-ohalsa-41-procent-hogre-risk-for-kvinnor
Gallup. (2021). Global Emotions Report. https://www.gallup.com/analytics/349280/gallup-
global-emotions-report.aspx
Gazzaniga, M., Irvy, R. B., & Mangun, G. R. (2013). Cognitive neuroscience: The biology of
the mind (4th ed.). W. W. Norton and Company.
Gruebner, O., Rapp, M. A., Adli, M., Kluge, U., Galea, S., & Heinz, A. (2017). Cities and
mental health. Dtsch Arztebl International, 114(8), 121–127.
https://doi.org/10.3238/arztebl.2017.0121
21
Gullone, E. (2000). The Biophilia Hypothesis and Life in the 21st Century: Increasing Mental
Health or Increasing Pathology? Journal of Happiness Studies, 1(3), 293–322.
https://doi.org/10.1023/A:1010043827986
Hanoch, Y., & Vitouch, O. (2004). When less is more: Information, emotional arousal and the
ecological reframing of the Yerkes-Dodson law. Theory & Psychology, 14, 427–452.
https://doi.org/10.1177/0959354304044918
Hansen, M. M., Jones, R., & Tocchini, K. (2017). Shinrin-yoku (Forest bathing) and nature
therapy: A state-of-the-art review. International Journal of Environmental Research
and Public Health, 14(8). https://doi.org/10.3390/ijerph14080851
Hassan, A., Tao, J., Li, G., Jiang, M., Aii, L., Zhihui, J., Zongfang, L., & Qibing, C. (2018).
Effects of walking in bamboo forest and city environments on brainwave activity in
young adults. Evidence-Based Complementary and Alternative Medicine, 2018.
https://doi.org/10.1155/2018/9653857
Hjortskov, N., Rissén, D., Blangsted, A. K., Fallentin, N., Lundberg, U., & Søgaard, K. (2004).
The effect of mental stress on heart rate variability and blood pressure during
computer work. European Journal of Applied Physiology, 92(1), 84–89.
https://doi.org/10.1007/s00421-004-1055-z
Hodson, C. B., & Sander, H. A. (2017). Green urban landscapes and school-level academic
performance. Landscape and Urban Planning, 160, 16–27.
https://doi.org/10.1016/j.landurbplan.2016.11.011
Holter, N. J. (1961). New method for heart studies. Science, 134(3486), 1214–1220.
https://doi.org/10.1126/science.134.3486.1214
Igarashi, M., Miwa, M., Ikei, H., Song, C., Takagaki, M., & Miyazaki, Y. (2015). Physiological
and psychological effects of viewing a kiwifruit (Actinidia deliciosa ‘Hayward’)
orchard landscape in summer in Japan. International Journal of Environmental
22
Research and Public Health, 12(6), 6657–6668.
https://doi.org/10.3390/ijerph120606657
Joung, D., Kim, G., Choi, Y., Lim, H., Park, S., Woo, J. M., & Park, B. J. (2015). The prefrontal
cortex activity and psychological effects of viewing forest landscapes in autumn
season. International Journal of Environmental Research and Public Health, 12(7),
7235-7243. https://doi.org/10.3390/ijerph120707235
Jöbsis, F. F. (1977). Noninvasive, infrared monitoring of cerebral and myocardial oxygen
sufficiency and circulatory parameters. Science, 198(4323), 1264–1267.
https://doi.org/10.1126/science.929199
Kaplan, S. (1995). The restorative benefits of nature: Toward an integrative framework.
Journal of Environmental Psychology, 15(3), 169–182.
https://doi.org/10.1016/0272-4944(95)90001-2
Kellert, S. R., & Wilson, E. O. (1993). The biophilia hypothesis. Island press.
Klein, L. C., & Corwin, E. J. (2002). Seeing the unexpected: How sex differences in stress
responses may provide a new perspective on the manifestation of psychiatric
disorders. Current Psychiatry Reports, 4(6), 441–448.
https://doi.org/10.1007/s11920-002-0072-z
Kobayashi, H., Ishibashi, K., & Noguchi, H. (1999). Heart rate variability; An index for
monitoring and analyzing human autonomic activities. Applied Human Science,
18(2), 53–59. https://doi.org/10.2114/jpa.18.53
Kobayashi, H., Song, C., Ikei, H., Park, B.-J., Kagawa, T., & Miyazaki, Y. (2019). Combined
effect of walking and forest environment on salivary cortisol concentration. Frontiers
in Public Health, 12, 376. https://doi.org/10.3389/fpubh.2019.00376
23
Kobayashi, H., Song, C., Ikei, H., Park, B.-J., Lee, J., Kagawa, T., & Miyazaki, Y. (2018).
Forest walking affects autonomic nervous activity: A population-based study.
Frontiers in Public Health, 278. https://doi.org/10.3389/fpubh.2018.00278
Kondo, M. C., Jacoby, S. F., & South, E. C. (2018). Does spending time outdoors reduce
stress? A review of real-time stress response to outdoor environments. Health &
Place, 51, 136–150. https://doi.org/10.1016/j.healthplace.2018.03.001
Largo-Wight, E., O’Hara, B. K., & Chen, W. W. (2016). The efficacy of a brief nature sound
intervention on muscle tension, pulse rate, and self-reported stress: Nature contact
micro-break in an office or waiting room. Health Environments Research & Design
Journal, 10(1), 45–51. https://doi.org/10.1177/1937586715619741
Lee, J., Park, B.-J., Tsunetsugu, Y., Ohira, T., Kagawa, T., & Miyazaki, Y. (2011). Effect of
forest bathing on physiological and psychological responses in young Japanese male
subjects. Public Health, 125(2), 93–100. https://doi.org/10.1016/j.puhe.2010.09.005
Lee, J., Tsunetsugu, Y., Takayama, N., Park, B. J., Li, Q., Song, C., Komatsu, M., Ikei, H.,
Tyrväinen, L., Kagawa, T., & Miyazaki, Y. (2014). Influence of forest therapy on
cardiovascular relaxation in young adults. Evidence-Based Complementary and
Alternative Medicine, 2014. https://doi.org/10.1155/2014/834360
Lim, Y., Kim, J., Khil, T., Yi, J., & Kim, D. (2021). Effects of the forest healing program on
depression, cognition, and the autonomic nervous system in the elderly with cognitive
decline. Journal of People, Plants, and Environment, 24(1), 107–117.
https://doi.org/10.11628/ksppe.2021.24.1.107
Mao, G. X., Lan, X. G., Cao, Y. B., Chen, Z. M., He, Z. H., Lv, Y. D., Wang, Y. Z., Hu, X. L.,
Wang, G. F., & Yan, J. (2012). Effects of short-term forest bathing on human health in
a broad-leaved evergreen forest in Zhejiang Province, China. Biomedical and
Environmental Sciences, 25(3), 317–324. https://doi.org/10.3967/0895-
3988.2012.03.010
24
McEwen, B. S., & Gianaros, P. J. (2010). Central role of the brain in stress and adaptation:
Links to socioeconomic status, health, and disease. Annals of the New York Academy
of Sciences, 1186(1), 190–222. https://doi.org/10.1111/j.1749-6632.2009.05331.x
Mello, A. F., Mello, M. F., Carpenter, L. L., & Price, L. H. (2003). Update on stress and
depression: The role of the hypothalamic-pituitary-adrenal (HPA) axis. Brazilian
Journal of Psychiatry, 25(4), 231–238. https://doi.org/10.1590/s1516-
44462003000400010
Michie, S. (2002). Causes and management of stress at work. Occupational and
Environmental Medicine, 59(1), 67–72. https://doi.org/10.1136/oem.59.1.67
Miyazawa, T., Horiuchi, M., Komine, H., Sugawara, J., Fadel, P. J., & Ogoh, S. (2013). Skin
blood flow influences cerebral oxygenation measured by near-infrared spectroscopy
during dynamic exercise. European Journal of Applied Physiology, 113(11), 2841-
2848. https://doi.org/10.1007/s00421-013-2723-7
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & The PRISMA Group. (2009). Preferred
reporting items for systematic reviews and meta-analyses: The PRISMA statement.
PLoS Med 6(7): e1000097. https://doi.org/10.1371/journal.pmed.1000097
Mulhall, A. (1996). Cultural discourse and the myth of stress in nursing and medicine.
International Journal of Nursing Studies, 33(5), 455–468.
https://doi.org/10.1016/0020-7489(96)00005-3
Nagasawa, Y., Ishida, M., Komuro, Y., Ushioda, S., Hu, L., & Sakatani, K. (2020).
Relationship between cerebral blood oxygenation and electrical activity during mental
stress tasks: Simultaneous measurements of NIRS and EEG. Oxygen Transport to
Tissue XLI, 1232, 99–104. http://doi.org/10.1007/978-3-030-34461-0_14
Oh, B., Lee, K. J., Zaslawski, C., Yeung, A., Rosenthal, D., Larkey, L., & Back, M. (2017).
Health and well-being benefits of spending time in forests: Systematic review.
25
Environmental Health and Preventive Medicine, 22(1), 1–11.
https://doi.org/10.1186/s12199-017-0677-9
Ouzzani, M., Hammady, H., Fedorowicz, Z., & Elmagarmid, A. (2016). Rayyan—A web and
mobile app for systematic reviews. Systematic Reviews, 5(1), 210.
https://doi.org/10.1186/s13643-016-0384-4
Park, B. J., Tsunetsugu, Y., Ishii, H., Furuhashi, S., Hirano, H., Kagawa, T., & Miyazaki, Y.
(2008). Physiological effects of Shinrin-yoku (taking in the atmosphere of the forest)
in a mixed forest in Shinano Town, Japan. Scandinavian Journal of Forest Research,
23(3), 278–283. https://doi.org/10.1080/02827580802055978
Park, B. J., Tsunetsugu, Y., Kasetani, T., Hirano, H., Kagawa, T., Sato, M., & Miyazaki, Y.
(2007). Physiological effects of Shinrin-yoku (taking in the atmosphere of the forest) -
Using salivary cortisol and cerebral activity as indicators. Journal of Physiological
Anthropology, 26(2), 123–128. https://doi.org/10.2114/jpa2.26.123
Park, B. J., Tsunetsugu, Y., Kasetani, T., Kagawa, T., & Miyazaki, Y. (2010). The physiological
effects of Shinrin-yoku (taking in the forest atmosphere or forest bathing): Evidence
from field experiments in 24 forests across Japan. Environmental Health and
Preventive Medicine, 15(1), 18–26. https://doi.org/10.1007/s12199-009-0086-9
Park, B. J., Tsunetsugu, Y., Kasetani, T., Morikawa, T., Kagawa, T., & Miyazaki, Y. (2009).
Physiological effects of forest recreation in a young conifer forest in Hinokage Town,
Japan. Silva Fenn, 43(2), 291–301. https://doi.org/10.14214/sf.213
Porges, S. W. (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143.
https://doi.org/10.1016/j.biopsycho.2006.06.009
Ridner, S. H. (2004). Psychological distress: Concept analysis. Journal of Advanced Nursing,
45(5), 536–545. https://doi.org/10.1046/j.1365-2648.2003.02938.x
26
Roberts, H., van Lissa, C., Hagedoorn, P., Kellar, I., & Helbich, M. (2019). The effect of short-
term exposure to the natural environment on depressive mood: A systematic review
and meta-analysis. Environmental Research, 177, 108606.
https://doi.org/10.1016/j.envres.2019.108606
Selye, H. (1956). The stress of life. McGraw-Hill
Seo, S.-H., Lee, J.-T., & Crisan, M. (2010). Stress and EEG. Convergence and Hybrid
Information Technologies, 27. http://doi.org/10.5772/9651
Shuda, Q., Bougoulias, M. E., & Kass, R. (2020). Effect of nature exposure on perceived and
physiologic stress: A systematic review. Complementary Therapies in Medicine, 53,
102514. https://doi.org/10.1016/j.ctim.2020.102514
Song, C., Ikei, H., Kagawa, T., & Miyazaki, Y. (2019a). Effects of walking in a forest on young
women. International Journal of Environmental Research and Public Health, 16(2),
229. https://doi.org/10.3390/ijerph16020229
Song, C., Ikei, H., Kagawa, T., & Miyazaki, Y. (2019b). Physiological and psychological effects
of viewing forests on young women. Forests, 10(8), 635.
https://doi.org/10.3390/f10080635
Song, C., Ikei, H., Kagawa, T., & Miyazaki, Y. (2020). Effect of viewing real forest landscapes
on brain activity. Sustainability, 12(16), 6601. https://doi.org/10.3390/su12166601
Song, C., Ikei, H., & Miyazaki, Y. (2018). Physiological effects of visual stimulation with forest
imagery. International Journal of Environmental Research and Public Health, 15(2),
213. https://doi.org/10.3390/ijerph15020213
Staats, H., Jahncke, H., Herzog, T. R., & Hartig, T. (2016). Urban options for psychological
restoration: Common strategies in everyday situations. PLoS One, 11(1), e0146213.
https://doi.org/10.1371/journal.pone.0146213
27
Stigsdotter, U. K., Corazon, S. S., Sidenius, U., Kristiansen, J., & Grahn, P. (2017). It is not all
bad for the grey city–A crossover study on physiological and psychological restoration
in a forest and an urban environment. Health & Place, 46, 145–154.
https://doi.org/10.1016/j.healthplace.2017.05.007
Takayama, N., Morikawa, T., & Bielinis, E. (2019). Relation between psychological
restorativeness and lifestyle, quality of life, resilience, and stress-coping in forest
settings. International Journal of Environmental Research and Public Health, 16(8),
1456. https://doi.org/10.3390/ijerph16081456
Thomas, G. G., & Lena, E. (2010). Chronic stress and the hpa axis: Clinical assessment and
therapeutic considerations. The Standard, 9(2), 1–12.
https://www.pointinstitute.org/wp-
content/uploads/2012/10/standard_v_9.2_hpa_axis.pdf
Tsunetsugu, Y., Lee, J., Park, B. J., Tyrväinen, L., Kagawa, T., & Miyazaki, Y. (2013).
Physiological and psychological effects of viewing urban forest landscapes assessed by
multiple measurements. Landscape and Urban Planning, 113, 90–93.
https://doi.org/10.1016/j.landurbplan.2013.01.014
Tsunetsugu, Y., & Miyazaki, Y. (2005). Measurement of absolute hemoglobin concentrations
of prefrontal region by near-infrared time-resolved spectroscopy: Examples of
experiments and prospects. Journal of Physiological Anthropology and Applied
Human Science, 24(4), 469–472. https://doi.org/10.2114/jpa.24.469
Ulrich, R. S. (1981). Natural versus urban scenes: Some psychophysiological effects.
Environment and Behavior, 13(5), 523–556.
https://doi.org/10.1177/0013916581135001
Ulrich, R. S. (1993). Biophilia, biophobia, and natural landscapes. Island Press
Ulrich, R. S., Simons, R. F., Losito, B. D., Fiorito, E., Miles, M. A., & Zelson, M. (1991). Stress
recovery during exposure to natural and urban environments. Journal of
28
Environmental Psychology, 11(3), 201–230. https://doi.org/10.1016/S0272-
4944(05)80184-7
Wilson, E. O. (1984). Biophilia. Harvard University Press.
World Health Organization. (2022, February). Self-care interventions for health
https://www.who.int/health-topics/self-care#tab=tab_1
Yao, W., Zhang, X., & Gong, Q. (2021). The effect of exposure to the natural environment on
stress reduction: A meta-analysis. Urban Forestry & Urban Greening, 57, 126932.
https://doi.org/10.1016/j.ufug.2020.126932
Yu, Y. M., Lee, Y. J., Kim, J. Y., Yoon, S. B., & Shin, C. S. (2016). Effects of forest therapy
camp on quality of life and stress in postmenopausal women. Forest Science and
Technology, 12(3), 125-129. https://doi.org/10.1080/21580103.2015.1108248
