
International Journal of
Environmental Research
and Public Health
Article
Assessing the Effects of Nature on Physiological States Using
Wearable Technologies
Dannie Fu 1, Natalia Incio Serra 2, Hubert Mansion 3, Emilia Tamko Mansion 3 and Stefanie Blain-Moraes 2,*
1 Department of Biomedical Engineering, McGill University, Montreal, QC H3A 2B4, Canada;
dannie.fu@mail.mcgill.ca
2 School of Physical & Occupational Therapy, McGill University, Montreal, QC H3G 2M1, Canada;
natalia.incioserra@mail.mcgill.ca
3 L’Université Dans la Nature, Montreal, QC H1V 1H6, Canada; hubert.tlmvdn@gmail.com (H.M.);
emilia@unature.org (E.T.M.)
* Correspondence: stefanie.blain-moraes@mcgill.ca
Citation: Fu, D.; Serra, N.I.; Mansion,
H.; Mansion, E.T.; Blain-Moraes, S.
Assessing the Effects of Nature on
Physiological States Using Wearable
Technologies. Int. J. Environ. Res.
Public Health 2022, 19, 1231. https://
doi.org/10.3390/ijerph19031231
Academic Editor: Paul B. Tchounwou
Received: 7 December 2021
Accepted: 20 January 2022
Published: 22 January 2022
Publisher’s Note: MDPI stays neutral
with regard to jurisdictional claims in
published maps and institutional affil-
iations.
Copyright: © 2022 by the authors.
Licensee MDPI, Basel, Switzerland.
This article is an open access article
distributed under the terms and
conditions of the Creative Commons
Attribution (CC BY) license (https://
creativecommons.org/licenses/by/
4.0/).
Abstract: Nature therapy and forest bathing (FB) have been shown to have quantifiable positive
effects on human health, but the physiological effects of a guided interactive nature activity remain
unexplored. Autonomic nervous system responses to a guided nature walk (Nature Break) were
assessed through the continuous measurement of the electrodermal activity (EDA), fingertip temper-
ature, and heart rate (HR) of n = 48 participants, using a wearable sensor. Psychological distress was
assessed before and after the activity using the Profile of Mood States (POMS) for n = 38 (24 females,
14 males, mean age = 43.55 ± 11.61 years) participants. The negative dimensions of POMS decreased
and the positive (vigor) dimensions increased following a Nature Break. Significant differences were
found across all of the physiological features, with some differences occurring between the morning
and afternoon groups and between different days. The participants’ mean HR decreased throughout
the Nature Break. Our results suggest that interactive nature activities have positive psychological
benefits and demonstrate the feasibility of using wearable sensors to monitor physiological responses
in a naturalistic forest bathing activity.
Keywords: forest bathing; autonomic nervous system; profile of mood states; wearable technology;
forest therapy
1. Introduction
In 2018, Northern America was the most urbanized geographic region in the world
with 82% of its population living in urban areas. Globally, 55% of the world’s population
was living in urban areas in 2018 with this number projected to reach 68% by 2050 [1]. Rapid
urbanization poses many socio-economic, environmental, and psychological challenges,
such as increased levels of crime, air and water pollution, and the psychological stressors
that are associated with higher levels of density and diversity of cities. The environmental
particulate matter that is associated with decreased air quality may be related to cardiovas-
cular deaths and asthma and noise exposure may be associated with hearing impairment,
hypertension, and ischemic heart disease [2].
Over the past few decades, there has been growing interest surrounding the impacts
of natural environments on human health and well-being. In 1982, the term shinrin-yoku,
also known as forest bathing (FB), was coined by the Ministry of Agriculture, Forestry
and Fisheries of Japan to describe the practice of immersing oneself in a forest environ-
ment using all five senses in order to improve one’s mental and physical health [3–5].
The accumulating evidence has demonstrated that exposure to nature has quantifiable
positive effects on the psychological and physiological health of human beings [3,5–7]. In
particular, the act of immersing oneself in a forest has been associated with decreased blood
pressure in comparison to immersion in urban environments [4,8]; significant decreases in
Int. J. Environ. Res. Public Health 2022, 19, 1231. https://doi.org/10.3390/ijerph19031231
https://www.mdpi.com/journal/ijerph

Int. J. Environ. Res. Public Health 2022, 19, 1231
2 of 16
physiological biomarkers of stress such as salivary cortisol and alpha-amylase [9,10]; and
improvements in psychological measures of tension, anger, fatigue, anxiety, confusion, and
depression [4,11–13].
Research in this field has been translated directly into various health initiatives, as
FB and nature walks have become increasingly popular as methods of promoting active
lifestyles and improving overall wellness [14] and have started being prescribed by doctors
and recommended by federal, provincial and local associations. On the global scale, Health
Parks Healthy People (HPHP) is a movement that is working alongside many large groups,
such as the National Park Service in the United States and Ontario Parks in Canada, to
advocate the physical and mental benefits of nature. For example, Mood Walks is a program
led by the Canadian Mental Health Association, Ontario, that promotes nature walks to
improve physical and mental health.
Research to date has provided psychological and physiological evidence regarding the
positive impacts of FB and nature walks. To date, most physiological studies have primarily
assessed the effects of natural environments on cardiovascular measures, such as heart
rate, heart rate variability (HRV), and blood pressure [3–5,12,15–18]; neuroendocrine or
metabolic indexes, such as cortisol and adrenaline [5,19–21]; or immune and inflammatory
indexes, such as natural killer (NK) cells and tumor necrosis factor-alpha (TNF-α) [5,22–24].
Few studies have assessed the physiological effects of natural environments on electro-
dermal activity (EDA) or peripheral skin temperature, which directly reflect sympathetic
and parasympathetic nervous system activity and have been reliably connected to an
individual’s mental and emotional state [25–28].
Further, only a few studies have investigated purposeful engagement with the envi-
ronment during a nature therapy session or walking session [29–31]. Duvall (2013) looked
at the impact of cognitive engagement strategies, such as focusing on a sense (e.g., noticing
different sounds) or taking on a new role (e.g., imagining they are looking for inspiration
as an artist), on well-being and perception of the environment during outdoor walking
sessions. They found that only those in the engagement condition became more satisfied
with multiple aspects in their walking environment, such as general appearance, nature
sounds, and the variety of things to look at [30]. Furthermore, the engagement condition
was associated with self-reported significant increases in attention and decreased feelings
of frustration [29,30]. Similarly, Korpela et al. (2017) found that focused attention on
interactions with nature was associated with mood enhancement and self-reported positive
restoration [31].
Building upon these studies, we aimed to assess the in-situ effects of a 120-min guided
Nature Break activity that encouraged participants to deliberately engage with their envi-
ronment. In order to characterize the effects of this activity on an individual’s physiological
state, we recorded three signals that reflect sympathetic and parasympathetic activity:
EDA, fingertip temperature, and HR. We monitored these signals using a wearable sensor
and mobile application in order to obtain moment-by-moment measures of physiological
reactions that could be correlated with the specific activities in the forest. We also assessed
the effect of the Nature Break activity on participants’ self-reported mood states using the
POMS questionnaire. We hypothesized that a Nature Break would psychologically reduce
negative affect and increase positive affect, in accordance with previous studies. We also
hypothesized that the different activities in the forest would induce distinct and dissociable
physiological response profiles in Nature Break participants.
2. Materials and Methods
2.1. Description of the Nature Break Activity
2.1.1. L’Université dans la Nature
L’Université dans la Nature (UdN) is a non-profit organization that is located in
Montreal, Canada. UdN’s vision is to create stronger and more sustainable communities
and economies through solutions that are inspired by nature. The mission of L’UdN is to
equip the decision-makers of today and tomorrow (e.g., university students) with reliable

Int. J. Environ. Res. Public Health 2022, 19, 1231
3 of 16
and sustainable methods of decision-making, stimulating creativity and innovation, and
preventing stress through encounters with nature.
2.1.2. Nature Break
Nature Break is a guided immersion event in the forest that lasts approximately
120 min. The guide educates the participants about the neuroscientific findings of the
impacts of nature on human health and also aims to catalyze a personal connection with
nature. This activity seeks to generate awareness of the changes in mental and physiological
state that are induced by anchoring the reasons for these changes in a concrete experience
with measurable effects.
Nature Break was modelled on scientific research conducted in Scandinavia, the
United States and Southeast Asia [32–34]. This non-sporting and non-doctrinal activity is
framed by sensory exercises and a dissemination of scientific knowledge about the benefits
of nature that are within everyone’s reach.
Nature Break is comprised of five principal segments in the forest, which incorporate
various themes surrounding the five senses (e.g., touch, sight, hearing, etc.). Individuals
are invited to perform exercises that stimulate their senses in each segment; all segments
were designed to guide and stimulate the participant’s perception and sensory experience
of the forest. See Table 1 for a detailed description of the segments and exercises.
Table 1. Description of segments in the Nature Break activity.
Forest Activity Segment
1: Pre-forest
2: Sitting on stumps
3: Breathing
4: Old tree
5: Walking barefoot
6: Ferns
7: Pine tree refuge
8: Post-forest
Description
The participants fill out pre-forest POMS and don the wearable sensor.
The UdN guide invites the participants to understand the course of
time in forests and invites comparisons with the speed of life in the city.
The participants are standing. The UdN guide invites them to do a US
Army breathing exercise and to focus on smells. The guide explains
what scientists have found about certain particles emitted by trees and
their healing benefits. S/he will also discuss facts about the air that is
breathed in the city. The participants will practice a breathing exercise
that they can repeat on a daily basis.
The participants are invited to lie down directly on the ground. The
UdN guide explains the scientific discoveries that are related to the
body’s contact with the ground. The participants practice an exercise
that they can repeat on a daily basis. The participants are also invited
to grab a handful of dirt and to observe and smell the contents.
The participants are invited to remove their shoes and socks for the
duration of the walk to the next stop.
The participants are invited to observe and listen to their immediate
environment. The UdN guide explains Dr. Ulrich’s “View through the
window” study [35] and the uses of nature in hospitals since this
discovery. The participants practice an exercise that they can repeat on
a daily basis.
The guide explains the discoveries that demonstrate the positive
impact of nature on mental health and invites participants to find their
own refuge in this clearing. They stay here for a couple of minutes and
then meet the guide. The participants are invited to taste some resin
from the pine trees.
the guides lead a group discussion about the participants’ forest
experience. The participants fill out post-forest POMS.
Length (min.)
~10–20
~10–15
~10–15
~20
~5
~20–30
~10–15
~15–20
2.2. Participants
A total of 58 individuals participated voluntarily in this study through UdN’s Nature
Break program. Thirty-eight participants from two days of data collection completed

Int. J. Environ. Res. Public Health 2022, 19, 1231
4 of 16
the POMS questionnaire (n = 38, 24 females, 14 males, mean age = 43.55 ± 11.61 years);
and 48 participants across all of the days of the data collection period provided their
physiological data for analysis. All of the participants were fluent in English and French.
2.3. Study Site
The study was conducted in the autumn season on a private woodlot in Barnston Ouest,
Quebec, which is located in the Great Lakes–St. Lawrence forest region in Canada. This
forest region is characterized by a mix of deciduous and coniferous trees, including red pine
(Pinus resinosa), eastern white pine (Pinus strobus), and yellow birch (Betula alleghaniensis), as
well as some boreal species, such as the American beech (Fagus grandifolia) (Information
from https://www.sfmcanada.org/en/canada-s-forests1 accessed on 26 January 2021. The
private woodlot was located away from traffic and the city. The Nature Break activity took
place in the forest where participants were immersed in the forest setting and could not
hear or see traffic or buildings. Meteorological data was collected from a nearby station in
Coaticook (latitude: 45◦09 00.000 N, longitude: 71◦48 00.000 W).
2.4. Procedure
The study was conducted on three days during the last two weeks of September, 2020.
The maximum and minimum temperatures were 11 ◦C and −3.1 ◦C, 26.5 ◦C and 12.5 ◦C,
and 23.8 ◦C and 17.1 ◦C for days 1 through 3, respectively. Each day accommodated a
group of ten participants in the morning, followed by another group of ten participants in
the afternoon. Upon arrival at the site, participants were greeted by the co-founder of the
Nature Break program who gave them a brief overview of the study and instructed them to
fill out both the informed consent form and the POMS for the pre-forest assessment of their
psychological state. Subsequently, we introduced the physiological monitoring technology
to the participants, showing them how to wear the sensor, what data was being recorded,
and how to use the smartphone application. Participants then donned the physiological
sensor on their dominant hand and kept the paired smartphone in their purse, pocket, or
held in their hand for the remainder of Nature Break. Baseline physiological measures were
obtained from when the participant put the sensor on to just prior to starting the activity.
We briefly reiterated the goals and content of the Nature Break and the study to
the group of participants, prior to proceeding to the forest. The Nature Break activity
consisted of eight segments (Table 1). After the activities in the forest, the participants
were led to a field where they filled out the POMS for the post-forest assessment of their
psychological state. The Nature Break’s guides led a discussion with the entire group about
their experiences in the forest.
2.5. Data Collection
2.5.1. Psychological Measurements
The Profile of Mood States (POMS) was used as a measure of psychological distress.
The original tool consists of 30 adjectives which collectively measure six effective dimen-
sions: tension-anxiety (T), depression-dejection (D), anger-hostility (A), fatigue-inertia
(F), vigor-activity (V), and confusion-bewilderment (C). Each adjective is rated using a
5-point scale from 0 to 4. Our study uses an adapted version of the POMS, consisting of
18 adjectives [36].
2.5.2. Physiological Measurements
The autonomic nervous system (ANS) is composed of the parasympathetic and sym-
pathetic branches, otherwise known as the “rest or digest” and “fight or flight” systems.
Typically, excitation of the sympathetic nervous system and inhibition of the parasympa-
thetic nervous system occurs in response to states such as anxiety and stress [37], while
the opposite may occur during states of relaxation [38,39]. Changes in autonomic nervous
system (ANS) signals, such as EDA, HR, and skin temperature, are connected to internal
emotional or mental states [40,41].

Int. J. Environ. Res. Public Health 2022, 19, 1231
5 of 16
Three physiological signals reflecting ANS activity were collected from a wearable
device called the Triple-Physiology Sensor (TPS) (Thought Technology Ltd.©, Montreal,
Quebec, Canada). The TPS is worn on the fingertip and secured with Velcro wrap. It
collects three physiological signals: (1) electrodermal activity (EDA), (2) skin temperature,
and (3) blood volume pulse (BVP), which is used to derive heart rate (HR) and heart rate
variability (HRV).
Electrodermal Activity (EDA)
Electrodermal activity (EDA) reflects sympathetic innervation of the ANS and has
long been used as an indicator of emotional arousal to external or internal stimuli, such
as emotional thoughts, memory recall, or novel, startling, or threatening stimuli [38,42].
These stimuli may evoke electrodermal reactions (EDRs) which appear as transient peaks
in the EDA signal.
Fingertip Skin Temperature
Fingertip temperature is often used as a measure of sympathetic activity but it is
dependent on the subject’s body temperature. When the initial fingertip temperature is
above 33.2 ◦C, sympathetic innervation induces vasoconstriction causing less heat to be
radiated from the surface; however, below this temperature, vasoconstriction is not possible
and vasodilation occurs in response to the same sympathetic stimulus [38,42]. Fingertip
temperature has been shown to decrease in response to stimuli such as sudden noises,
fear, pain, and mental stimulation (such as motor imagery), and to increase during mental
relaxation [38,42,43].
Heart Rate (HR)
The heart is innervated by both the sympathetic and parasympathetic branches of the
ANS. Heart rate has been shown to decrease when an individual’s attention is focused
on the external environment and when sensory information gathering is high, it has been
shown to increase during internal focus or when the sensory input is dampened [38].
Higher HR is also associated with elevated states of stress and anxiety [44,45].
2.6. Data Analysis
The difference in psychological and physiological measures was assessed before and
after the Nature Break activity. Changes in the physiological signals were also assessed
across all of the segments of the activity that took place within the forest.
2.6.1. Psychological Measures Analysis
For the pre-post Nature Break psychological assessment, the mean POMS score for
each dimension was taken across all participants (n = 38). The pre-post significance was
computed using Wilcoxon signed-rank paired t-tests. The results were tested against the
Bonferroni-Holm adjusted alpha levels of 0.05 and 0.01.
2.6.2. Physiological Measures Analysis
First, each of the ANS signals were pre-processed in order to remove non-physiological
artifacts by applying smoothing filters (1D median filter, moving average filter), cubic
spline interpolation, and modality specific filters. A one Euro filter was applied to the EDA
signal; an exponential decay filter was applied to the skin temperature signal; and a cubic
smoothing spline was applied to the HR signals [46].
The signals were then segmented by forest activity and the following features were
extracted [47]: (1) The standard deviation of the slopes of the EDA was used to determine
the presence or absence of EDRs; (2) The median of the slopes of the skin temperature
was used to determine its general increasing/decreasing trend; and (3) The mean of the
slopes of the HR was used to determine its general increasing/decreasing trend. Figure 1 is
an example of the physiological signals that were derived from two of the forest activity
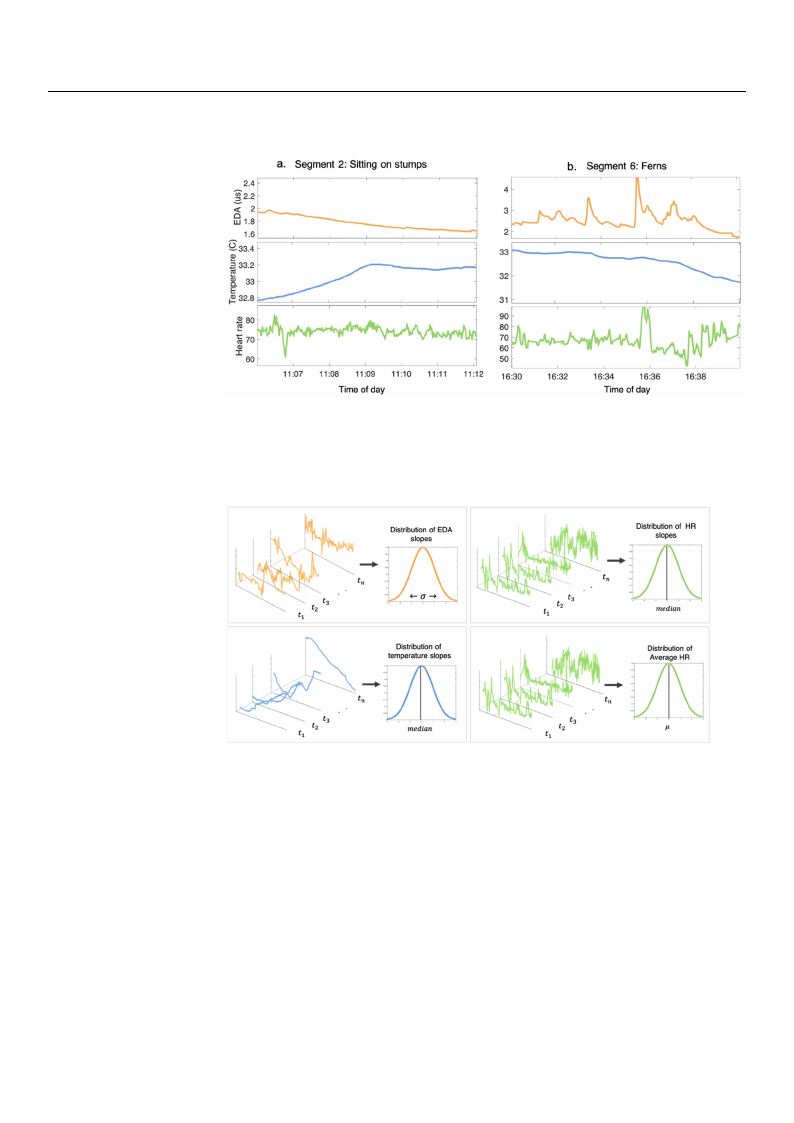
TThheesisginganlaslswwereerethtehnensesgemgmenetnetdedbybfyofroesret satcaticvtiitvyityanadntdhethfoelfloolwloiwnginfegafteuarteusrwesewreere
eexxtrtarcatcetded[4[74]7:](:1()1T) hTehestsatnadnadradrdedveivatiaiotinoonfotfhtehselosploepseosfothf ethEeDEADwAaws ausseudsetdo dtoetdeermterinmeine
ththeepprerseesnenceceororabasbesnecneceofoEf DEDRsR;s(;2()2T)hTehme medeidaniaonfothf ethselosploeps eosf othfethsekisnktinemtepmerpaetruarteuwreaws as
uusesdedtotoddeteetremrminieneitsitsgegneenrearlailnicnrceraesainsign/gd/edcerecaresainsgintgretnredn; dan; adn(d3)(3T)hTehmeemaneaonf tohfethsleospleospes
Int. J. Environ. Res. Public Health 2022o, 1of9ft,h1th2e3e1HHRRwwasasusuesdedtotodedtetremrminieneitsitgsegneenrearlailncinrecaresiansgin/dge/dcreecarseiansgintgretnrden. dFi.gFuirgeu1reis16aiosnf 1a6n
eexxaammppleleofotfhtehephpyhsyisoiloolgoigciaclasligsinganlasltshtahtawt ewrerdeedrievreivdefdrofmromtwtowoof othfethfoerfeosrteascttaivcittiyvisteygs-eg-
mmenentst;s;FFigiugurere2 2illiullsutsrtartaetsesthtehefefaetautruersetshtahtawt ewrereexetrxatcrtaecdtefdorfoeracehacphhpyhsiyosloiogliocgailcsailgsniaglnal
fsrfeorgommmeeanecatshc;hfFofirogerusertseat2catciilvtliuivtsyittyrsaestgeemgs mtehneetn.fte.atures that were extracted for each physiological signal
from each forest activity segment.
FFiiggiguuurreere1.1E. xEaxmamplpelseosoffoppfrrpee-r-pepr-ropocrceoescssesesedsdeEdEDDEAAD, ,sAks,kinsinkteitnmemtpeepmreaprtaeutrruaert,eua,rnaedn, daHnRHdsRHigsRnigasnlisagdlnsuadrlisunrdgiunagrcitnaivgcittiayvcisttieyvgismteygesn-etgs -
minmetnehntestsifnoinrtehtsehte.fo(fraoe)rs.etTs. t(h.ae()a.p)T.ahTrethipeciappraatirnctitipc’siapnsaitgn’snt’sasilgssnigdanulsardlisnugdruisnregignsmgegesnmegtem2ntewn2htwe2nhwetnhheethynetwyheewryewrseietsrtiietntsignitgitnininagacinicricarlceclierocnle
otronenetrtserteeuemstsuptmus,mplisps,tsel,inslitisentngeinntiogntgthoetotghtuehiegduegiutdaieldkte.al(tkba.l)k.(b.T()hb. eT).phTeahrpteiacpritpaicartinpicta’ispnats’insgtns’siaglsnsiagdlnsuardlisundrgiunsrgeignsmgegesmnegtem6n,tew6n,htwe6n,hwethnheeyn
stwshettsiheneemryneseoyuswrsolwyiiret,ytressietnrusiemtsgciihmstiutintialuntisai,lgnissb,guoisenucudihnnacohdaabfsaefbsdesneordsdnouofsntouh.fdfneDefrdapeunnrarsdnin.tntsDdteg.hruDtenshresupiegnraompgitnntaeesgtenrhtnegsteersm6ngl,oeesmpnanovaettnrenh6tsteit,.ch6plieep,aaaplrvetnaiaetcrsvsti.piewcasi.epnrtaesnawtsekwreedeartesokafesodkceutdos fotooncfuvosacruoionsuovsnasrevionausroisoruys
Figure 2. The average slopes of the EDA, HR, and skin temperature were computed across 60 s
sFlFiigdiguinurgerew22.in.TdThoheweasavivenerreaaaggcehesfsloolorpepesestsaocoftfitvhtihetyeEsEDeDgAmA, ,eHnHRt.R,F,aeanantdudrseskskinienxttetremamcptpeederrafatoutrurereaewcwheefroerereccostomampcptuiuvteittedydsaeacgcrormosesssn6t60: 0ss
sstlssailtdniadindnidangragdwrwddiniedndvedoivaowitwaisotsinioninnoefoeatfachchtehhfEeofDoErerADesstAstalaocsctlpoitveipvsite,iytsmy,semsedegegimadmnieaenonntf.tot.FhfFeeteahatteeutmutrerepmeseseprexaexttrurtarartaceuctertaeednddafnofHordrReHeasacRlchohspflfoeoosrpre,eesmsstt,eamaaccntetiviaHvintiRtyHy. sRseeg.gmmeenntt::
standard deviation of the EDA slopes, median of the temperature and HR slopes, mean HR.
The physiological measures were first analyzed across all of the participants, not tak-
ing inTtTohheaecpcphohyuysnisotioltohlgoeigceiafcflaemlcmtesaeosafustruheresetwsimwereee-rofiefr-fsditrasaytnaoanrlyaazlmyedzbeiaedcnratoctsresomasslpleaorlflatothufertehp.eaWrpteiacrtithpiecaniptcaso,nntdso,utntcaotketditnagk-
tiwnintoog cainocctnootruaonclctoatnhuaenlteytfshfeeescteisfnfoeofcrttdhs eorftittmhoeed-toeimtfe-derm-aoyifn-oderatayhmeobreiaeffmnetcbtiseemnotfptetehrmaetpuaemrera.btiWuenreett.htWeemnepcthoerenandtucocretnedaduntcdwteod
tcitomwneot-rocflo-adnnatrayol.ylWsaenesadilniyvsoiedrsdedeinrtthooerddaeentraeltryomsidisneietnetrohmedieanfyefe1tchtpseaoretfficftehipcetasanmtosfb(itnehn=et1at6em)mabpniedenrdtatatueyrme2 paenrdat3tuimpreaer-a-onfd-
tditcaiimyp.aeWn-otesf-dd(niavy=id. 3We3d)etdhdueiveaidtnoeadltyhtsehisesiganntoaiflidycasiynst1indptiofafredtriaecyinpc1aenpitansrt(ihncei=paa1mn6)tbsaie(nndt=dte1am6y)p2aenardantdduar3eyps2abraetntiwcdipe3eanpnatsr-
t(hntiec=siep3at3wn) todsug(enrot=uop3tsh3)e[3ds8iu,g4en2]it.fioKctaihsnettlesdrigiefnfteiafrlie.cna(1cn9et9id8n)iftrfheepreoanrmcteebdiientnhttahteethmaempsebyrimaetnputartehesmebtipectewsrtaiemteunureltashteibosenetwtowfeeon
gtrhoeuspestw[3o8,g4r2o].uKpsis[t3le8r,4e2t]a. lK. i(s1t9le9r8e) treapl.o(r1t9e9d8)thraetptohretesdymthpatathetsicymstipmatuhleattiiconstiomf ufilnagtieorntipof
temperature is dependent on the overall body temperature. Across day 1 participants,
the average fingertip temperature was 18.8 ◦C, whereas for day 2 and 3 participants, the
average was 30.0 ◦C. In this paper, we will refer to day 1 and the day 1 participants as a “cool
day” and the “cool day participants”, and days 2 and 3 and their respective participants as
“warm days” and the “warm day participants”.
We assessed the effect of the time-of-day based on whether the participants were in the
morning or afternoon group. Day 2 and 3 participants had their associated POMS scores

Int. J. Environ. Res. Public Health 2022, 19, 1231
7 of 16
taken account in the statistical model (day 1 participants did not have psychological data
collected to be included in this analysis).
2.6.3. Determining the Physiological Effect of Nature Break
To determine if there was a significant difference in the physiological signals that
were recorded before and after the Nature Break, a paired samples t-test was used for the
normally distributed physiological features and a Wilcoxon signed-rank test was used for
the non-normally distributed features. A mixed model was used to assess the significance
differences across the forest activity segments, with the segment as a fixed effect. A mixed-
model approach was selected in order to take into account the missing data that occurred
due to technical difficulties or unclean data.
Control Analysis 1: Ambient Temperature
The participants were divided into cool day (day 1) and warm day (day 2, 3) groups in
order to take into account the significant difference in ambient temperatures between these
days. The pre-post analysis and the analysis across the forest activity segments followed the
procedure that was described in Section 2.6.3; morning and afternoon group and pre-forest
POMS scores were not considered in this portion of the analysis.
Control Analysis 2: Time of Day
For both groups, a paired samples t-test or Wilcoxon signed rank test was used to
determine if there was a significant difference before and after the Nature Break for each
physiological signal. Independent t-tests were used on the pre-post difference in order
to assess the difference between the morning and afternoon groups. Welch’s t-test was
used when the variances were unequal and a Mann-Whitney U test was used for the
non-normally distributed features. We performed an Analysis of Covariance (ANCOVA)
in order to assess whether the means between the morning and afternoon groups were
significantly different post-forest, adjusting for the pre-forest measurements. A mixed
model was used in order to assess the significance differences across the forest activity
segments, with segment and time-of-day as fixed effects. For the day 2 and 3 participants
analysis, the ANCOVA also adjusted for pre-forest positive and negative POMS scores and
the mixed model accounted for the pre-forest rounded positive and negative POMS scores.
The p-values from the multiple comparisons between morning and afternoon groups
at each forest activity segment, as well as multiple comparisons between the segments,
were adjusted using the Bonferroni method.
3. Results
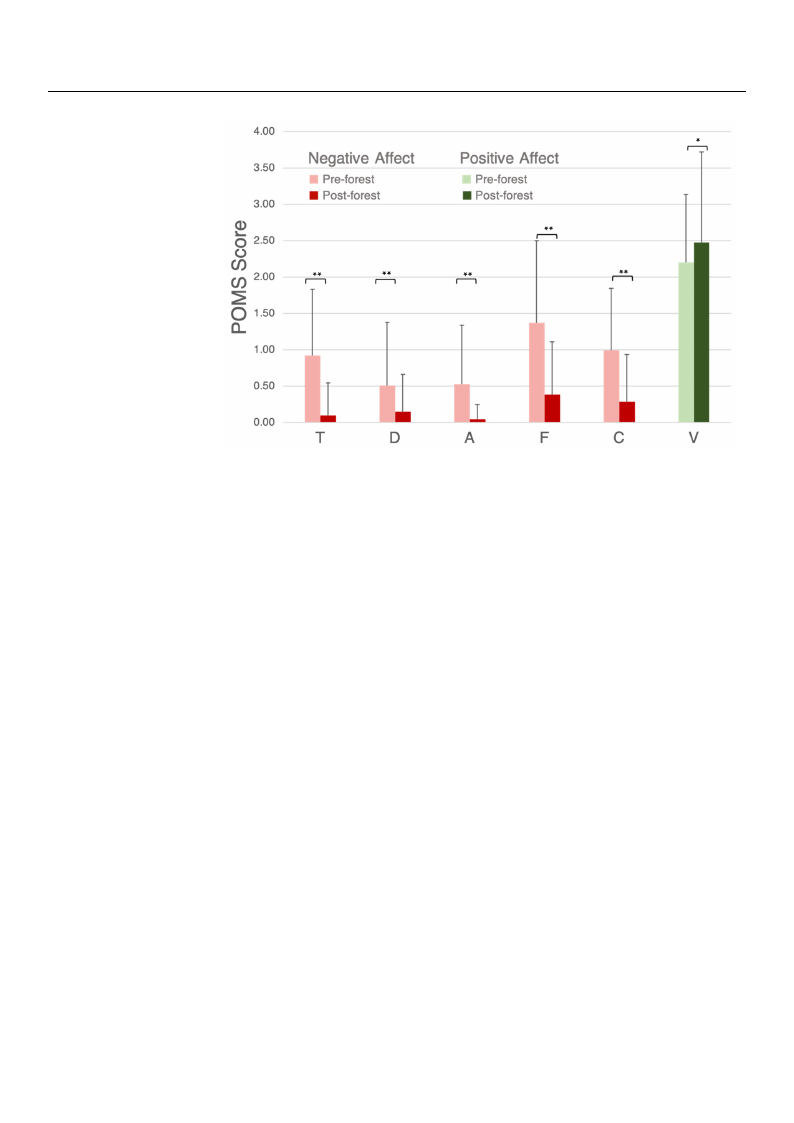
3.1. Forest Bathing Increases Positive Mood States and Decreases Negative Mood States
The average POMS scores for the negative mood states significantly decreased follow-
ing the Nature Break (p < 0.01), while the average POMS score for the positive mood state
(V) significantly increased following the Nature Break (p < 0.05) (Figure 3).
3.2. Purposeful Engagement with the Forest Induces Different Physiological Response Profiles
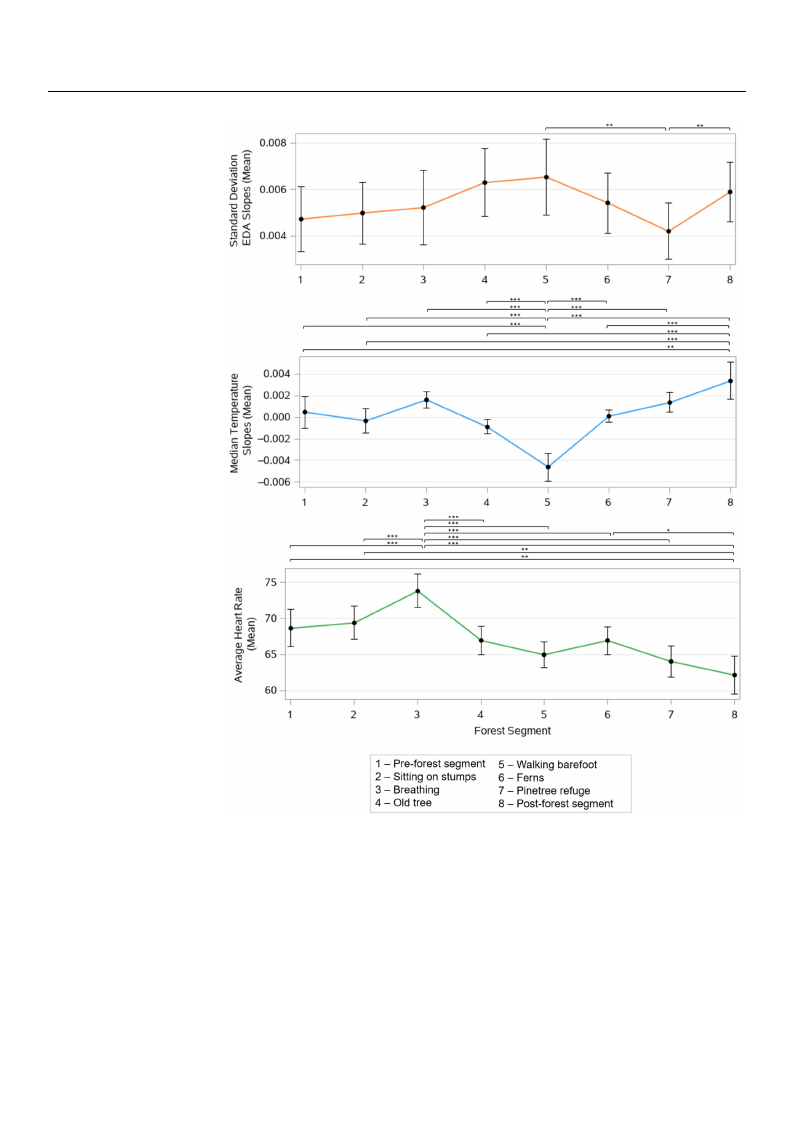
The FB segments had significant effects on all of the recorded physiological features,
including the mean standard deviation of the EDA slopes (p < 0.001 un-adjusted), mean of the
median skin temperature slopes (p < 0.001 un-adjusted), and mean of the average HR values
(p < 0.001 un-adjusted). Figure 4 shows the mean value with a 95% confidence interval of
these three measures across all of the segments and the Bonferroni adjusted p-values.
nt. J. Environ. Res. Public Health 2022, 19, x
Int. J. Environ. Res. Public Health 2022, 19, 1231
8
8 of 16
Figure 3. POMS results pre-forest and post-forest. T: tension and anxiety, D: depression and dejection,
FiguAr: ean3g.ePr OanMd Shorsetsiluitlyt,sFp: fraet-igfouree, sCt: aconndfupsoiosnt,-fVo:rveisgto.rT. :Sitgennisfiicoanntadnifdferaennxceiestayr,eDm:adrkeepdrewsisthion and d
tion*,pA<:0.a0n5gaenrd *a*npd<h0.o0s1t. ility, F: fatigue, C: confusion, V: vigor. Significant differences are ma
with * p < 0.05 and ** p < 0.01.
The participants experienced the largest sympathetic arousal, as manifested by their
3.2.EPDuAr,pdouserfiungl Ethnegaogldemtreenet(wseigtmh etnhte 4F)o, rbeasrteIfnoodtuwceaslkDinifgfe(rseengtmPehnyt s5i)o,loagndicaploRste-sfoproenstse Profil
(segment 8) activity segments, and the lowest sympathetic arousal during the pine tree
refTuhgee sFeBgmseengtm(seengmtsehnta7d).sTighenipfiinceantrteeerfefefucgtse soengmalelnot f(stehgemreencto7r)ddeemdopnhstyrsaiteodlothgeical feat
incllaurdgeinstgditfhfeeremnceeatno tshteabnadraefrodotdweavlkiaintigoanndofthtehpeoEstD-foAresstloacpteivsit(ypse<g0m.0en0t1s (upn<-a0.d01ju),sted), m
of tlhikeelmy deudeiatonthsekivnartioeums psteimrautluatriengslaoctpiveistie(spd<ur0in.0g0t1heulnat-taedr tjwusotseedg)m, eanntds. mean of the ave
HR3v),apliSunkeeisntrt(eepme<pre0efr.ua0gt0ue1re(ushenagd-matdehnejutlas7rt)ge,edasnt).rdaFtpeigoosufti-rnfeocrr4eeasssthe(osdewugrmsinetghnttehe8m)braeecaatntihviivntyaglasuecetgimvwiteyint(htsse,aglmi9k5eenl%yt confid
inteinrdviaclatoinf gthreelsaexatthiorne.e Tmheisafseuartuerseahcardosthseallalrogefstthreatseeogfmdeecnrtesasaenddurtihneg Bthoenbfaerrerfoonoti adjust
valwuealsk.ing activity (segment 3), likely reflecting focus and attention. The barefoot walking
activity demonstrated a significantly lower skin temperature slope than all of the other
segments, with a significance of p < 0.001. The post-forest activity segment (segment 8)
was also significantly different from all of the other segments, with p < 0.01; except for the
breathing (segment 3) and pine-tree refuge (segment 7) segments.
Heart rate was highest during the breathing activity and lowest during the post forest
activity segment. The breathing activity induced a significantly higher heart rate compared
to all of the other segments (p < 0.001) and, following this activity, there was a downward
trend of the mean HR. The mean of the median HR slopes showed no significant differences
across the segments.
3.3. Ambient Temperature Has an Effect on Skin Temperature and Heart Rate Response
For the cool day’s participants, the average standard deviation of the EDA slopes
and median of the skin temperature slopes were significantly higher following the Nature
Break (p ≤ 0.001, p < 0.05). For the warm days’ participants, the mean average HR was
significantly lower and the average median of the skin temperature slopes was significantly
higher following the Nature Break (p = 0.001, p < 0.05).
InItn. tJ.. JE. nEvnivroirno.nR. Rese.sP. PuublbilcicHHeaealtlhth22002222, ,1199, ,1x231
9 o9fof1716
Figure 4. Effect of forest bathing on the mean standard deviation of the EDA slopes, mean of the
mFeigduiarne t4e.mEpffeercattuofrefosrloepstesb,aathnidnmg eoanntahveemraegaenhsetaarntdraatredadcerovsiastaiollnfoorfestht eacEtiDviAtyssloegpmese,nmtse.a*npo<f 0th.0e5,
**mpe<di0a.n01t,em***pper<at0u.r0e01s,loBpoensf,earnrodnmi aedajnusatveedrapg-veahlueeasr.t rate across all forest activity segments. * p <
0.05, ** p < 0.01, *** p < 0.001, Bonferroni adjusted p-values.
Across the forest activity segments, participants from both the cool and warm days
exhibiTtehde spiagrntiicfiicpaannttds iefxfepreerniecenscefodrtahlel olafrtgheestpshyymsipoalothgeictaicl aferaotuusraels,.aTshmeamniefaenstaedvebryagteheHirR
wEaDsAth, eduhriignhgetshtedoulrdintgreteh(esebgrmeaetnhtin4g), abcatrievfiotyot(wseaglmkinengt(3se)gamndendte5m), oannsdtrpaotestd-faorgeesnt (esreagll-y
dmecerneta8s)inagctitvreitnydsefgomlloewntisn, gantdhitsheselgowmeesnttsfyomr pbaotthhettihcearcoouoslaal nddurwinagrtmhedpainyeptarereticreipfuagnets.
Tsheigsmseegnmt (esengtmweansts7ig).nTifihceapnitnlye tdriefeferreefnutgferosemgmalelnotf(tsheegmotehnetr7s)edgemmeonntsstfroartetdhethceoolalrdgaeyst’s
pdairftfiecriepnacnetstoantdhealblaorfetfhooetowthaelrksineggmanendtsth, eexpcoepstt-ffoorretshteapctriev-iftoyressetgamcteinvtitsy(pse<gm0.0en1)t,, lfiokretlhye
wdauremtodtahyes’vparairotuicsipsatinmtsu.lating activities during the latter two segments.

Int. J. Environ. Res. Public Health 2022, 19, 1231
10 of 16
The barefoot walking section (segment 5) induced the largest decrease in heart rate
for the cool day’s participants and was significantly different from the sitting on stumps
segment (segment 2) and the post-forest activity segment (segment 8) (p < 0.05); the latter
two segments demonstrated the largest increase in heart rate. In contrast, the barefoot
walking segment induced the largest increase in heart rate for the warm days’ participants
and was significantly different from all of the other segments, apart from the post-forest
activity segment (p < 0.05).
The cool day’s participants experienced a decrease in skin temperature during the
pre-forest and post-forest activity segments (segments 1 and 8), whereas the warm days’
participants experienced the largest increase in skin temperature during these segments.
Participants from all of the days experienced the largest or second largest decrease in
skin temperature during the barefoot walking segment (segment 5). This segment was
significantly different from all of the other segments for the warm days’ participants
(p < 0.001), and all of the other segments, except the pre-forest, old tree, and post-forest
activity segments (segments 1, 4, and 8), for the cool day’s participants (p < 0.05). These
differences are consistent with the expected effects due to the diverging vasoconstriction
and vasodilation response on hot and cold days, respectively.
3.4. Circadian Rhythms Affect Skin Temperature Response to Forest Bathing
The participants from all of the days demonstrated a significant difference between
morning and afternoon groups for the change in average median of the skin temperature
slopes pre-forest to post-forest (segment 1 to 8) (p ≤ 0.001). The afternoon had a smaller
change in skin temperature slopes post-forest from pre-forest than the morning group. Cool
day participants also saw a smaller difference in average heart rate post-forest from pre-forest
in the afternoon group (p < 0.05). No other features demonstrated a significant difference
between morning and afternoon groups in terms of pre-forest to post-forest differences.
Results of the ANCOVA showed no significant effect of time-of-day for the cool day’s
participants, meaning there was no significant difference between morning and afternoon
group post-forest when adjusted for the pre-forest measurements. For the warm days’
participants, the average median of the skin temperature slopes showed a significant effect
of time-of-day (p = 0.001). The afternoon group experienced less of an increase in skin
temperature post-forest than the morning group, with a significance of p = 0.001. The other
features for the warm days’ participants showed no significant difference between morning
and afternoon groups at the post-forest activity segment when adjusted for pre-forest
measurements, pre-forest POMS scores, and time-of-day.
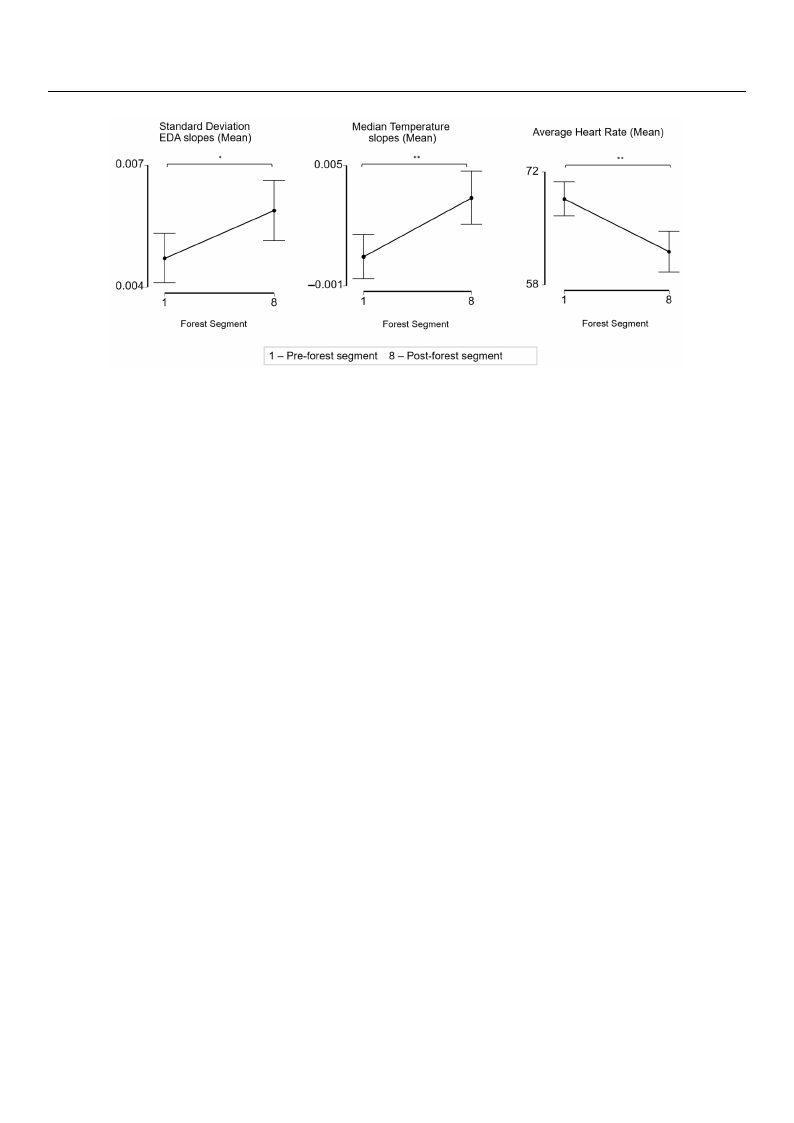
3.5. Participants Have Significant Physiological Differences Pre- and Post-Forest Bathing
To facilitate the comparison of the results from this study to those of previous research,
we also conducted a physiological analysis pre- and post-forest bathing. Across the par-
ticipants, the average of the standard deviation of the EDA slopes and the median skin
temperature slopes were significantly higher after the forest (p < 0.05, p < 0.01) and the
average heart rate was significantly lower after the forest (p < 0.01) (Figure 5).
Notably, the post-forest physiological data included a segment where participants
engaged in a discussion about their forest experience, in which several participants were
visibly emotional. This may have confounded the post-forest physiological signals, result-
ing in the “post” segment not being representative of “post” intervention, but of a final
active ingredient of the FB experience.
3.5. Participants Have Significant Physiological Differences Pre- and Post-Forest Bathing
To facilitate the comparison of the results from this study to those of previous re-
search, we also conducted a physiological analysis pre- and post-forest bathing. Across
the participants, the average of the standard deviation of the EDA slopes and the median
Int. J. Environ. Res. Public Health 2022, 19, 1231skin temperature slopes were significantly higher after the forest (p < 0.05, p11<o0f .1061) and
the average heart rate was significantly lower after the forest (p < 0.01) (Figure 5).
Figure 5. Effect of foFriegsut rbeat5h. inEgffoenct tohfe fmoreeasnt bstaatnhdinagrdodnetvhieatmioneaonf stthaenEdDarAd sdleovpieast,iomneoanf tohfethEeDmAesdloiapneste, mmpeearnatoufre
slopes, and mean avethraegme hedeaiarnt rtaetme fpreormatupre-sfolorpesetsa, catnivditmy eseagnmaevnetratogephoseta-rftoreastteafcrtoimvitpyrsee-gfomresntt.a*ctpiv<it0y.0s5e,g*m* pen<t0t.o01,
paired t-test and Wilpcoxsto-nfosriegsnt eadctrivaintky tseesgtm. ent. * p < 0.05, ** p < 0.01, paired t-test and Wilcoxon signed rank test.
4. DiscussiNonotably, the post-forest physiological data included a segment where participants
Thenegaaigmedoifnthaids istcuudssyiownaasbotoutetxhpeliorrfeortheset efxfpecetrsieonfcea,nininwtehriacchtisveev,egraulidpeadrtinciaptaunrets were
activitvyisoinblpyheymsiootliogniacla. lTahnisdmpasychhaovleogcoicnafloruenspdoednstehse. pPossytc-fhoorleosgtipchalylysi,otlhoegpicaarltsicigipnanlst,sresult-
showeidnga isnigtnhiefi“capnotstd”escergeamsenitnntohteibrenineggarteipvreeaseffnetcattiavnedoaf “spigonsitfi”cianntet rivnecnretiaosne,ibnutthoefira final
positivaecatifvfeecitnfgorlleodwieingt oafNthaetuFrBe eBxrpeaekri.eTnhce.physiological responses changed significantly
according to the different activities within the forest, with participants also experiencing
signific4a. nDtipshcuyssioiolongical differences before and after the Nature Break. Our results suggest
that interacTtihvee aniamtuoref athctisivsittuiedsyhawvaesptooseitxivpelopresytchheoeloffgeicctaslobfeannefiitnstearnadctdiveem, ognusidtreadtentahteure ac-
feasibitliivtyityofounsinpghywsieoalroagbilcealseannsdorps styocmhoolnoigtoicrapl hreysspioolnosgeisc.alPrseyscphoonlosegsicianllay,nathtueraplaisrttiiccipants
forest bshaothwinegd aactsiivgintyi.ficant decrease in their negative affect and a significant increase in their
ApgorsoitwiviengafbfeocdtyfoolfloewviidnegncaeNshaotuwres tBhraetaFkB. Tahnedpnhaytusiroelowgaiclkasl rpersopvoidnseeqsucahnatnifigaebdlesignifi-
physiocloangitclyalaacncdormdeinngtatlobtehneefidtisffteorehnutmaacntivbietiinesgsw, situhcihnatshreefdourecisnt,gwcoitrhtispoalrlteicvieplas,nHtsRa,lasnodexperi-
nblaotoudreepanrcectisinsvguitrsyeigi[nn3,ci5fri,ec1aa2sn,1ets4p,p4h8oy,s4sii9toi]vl.oeTghmicisaolosdtduifdsfteyartepenrsoc, evdsiedbceersefoafruseerstahnnedergaeafvttieidvreetnhmceeoNtohadtautsrtaeantBeirsne,taaekrn.adOcthuivaresresults
significant effects on physiological state. We found significant responses across all of the
four physiological ANS features during the various activities of the Nature Break, indicating
the potential for using a wearable sensor to monitor the effects of deliberate engagement
with nature.
Previous studies using the POMS questionnaire found that negative mood states im-
proved and positive mood states increased following a forest bathing and nature walk activ-
ity [4,8,11,49]. Other studies that have looked at purposeful engagement with nature have
also reported mood enhancement and overall satisfaction with the nature session [29–31].
Our findings support this trend as well, providing further evidence that an interactive,
guided nature activity can provide positive psychological experiences to humans. Cox,
Shanahan, et al. (2017) deconstructed the nature experiences of 1023 residents of an urban
population in the UK and found that a person’s connection to nature was positively corre-
lated with them spending time in nature [50,51]. By fostering a positive forest experience,
we anticipate that the Nature Break will similarly lead to participants spending more time
in a forest environment in the future.
Few studies have assessed the effect of forest bathing in-situ using wearable sen-
sors [25,49]. We showed that different activities conducted within the forest environment
have different physiological effects, which contribute to creating a dynamic, embodied
experience of immersion in nature. The participants across all of the days showed, in
general, a decreasing average heart rate across the forest activity segments. These results
are consistent with previous studies that have measured the effects of exposure to nature

Int. J. Environ. Res. Public Health 2022, 19, 1231
12 of 16
on heart rate [19,25,34,49,52,53]. EDA and peripheral skin temperature have received little
attention in the context of FB and nature walks and studies that have investigated EDA
did not report any significant findings [25,27]. Reeves et al. (2019) and Chen et al. (2018)
have used EDA to assess ANS activity, specifically as a measure of physiological stress and
arousal. Reeves et al. (2019) found no significant effects of site (wetland, control, urban)
on tonic or phasic EDA. Chen et al. (2018) did not report specifically on EDA alone, but
combined it with ECG measures to predict arousal and used HR and facial EMG to predict
the valence. Chen et al. (2018) also measured skin temperature in their study, however
they did not mention how it was used to contribute to their assessment of ANS activity. In
contrast, we saw significantly greater standard deviation of the EDA slopes—indicating
larger numbers of electrodermal reactions—and median of the skin temperature slopes
post-forest compared to pre-forest across all participants.
Across the forest activity segments, the participants from all of the days had the largest
sympathetic activity during the post-forest discussion (segment 8), old tree (segment 4),
and barefoot walking (segment 5) segments, as manifested by their EDA, likely due to the
various activities of touching, smelling, walking on dirt, and emotional discussion during
these segments. As well, a lower standard deviation of the EDA slopes was observed
during the pine tree refuge segment (segment 7), likely reflecting relaxation [38]. Peripheral
skin temperature saw the greatest rate of decrease during the barefoot walking segment
(segment 5) across all participants, likely reflecting the mental stimulation associated with
focusing on the barefoot walking task [38]. Higher rates of increase in the peripheral skin
temperature typically reflect mental relaxation and these were seen during segments such
as the deep breathing, pine tree refuge, and post-forest activity segments (segments 3, 7,
and 8) [38,43].
We found that the ambient temperature mainly had an effect on the skin temperature
and heart rate response. The largest rate of decrease in the heart rate occurred during the
barefoot walking segment (segment 5) for the cool day’s participants, however the warm
days’ participants experienced the largest rate of increase during this segment. Similarly,
the cool day’s participants showed a decrease in skin temperature during the pre-forest
and post-forest section (segment 1 and 8), but the warm days’ participants had the largest
increase in skin temperature during these segments. Differences in the rate of skin tempera-
ture change during the pre-forest and post-forest activity segments may be attributed to
the difference in the ambient temperatures of those days and the presence of direct sunlight
or overcast weather. The ambient temperature and other meteorological elements may also
affect the ground temperature, which could have an impact on HR response during the
barefoot walking segment [54]. In their study, Korhonen (2006) examined HR response
in men who were exposed to local cold exposure and whole-body cold exposure. They
found that local cold exposure to the feet increased the HR, while consistent whole-body
exposure to cold lowered the HR [55]. Similar effects may characterize the difference in the
HR responses in the cool day’s and warm days’ participants due to the variances in the air
and surface ground temperatures.
Circadian rhythms also affected skin temperature. The skin temperature in the af-
ternoon groups had a smaller change from pre-forest to post-forest. For the warm days’
participants, the afternoon groups’ skin temperatures increased less than the morning
groups’ at the post-forest activity segment. The limited amount of meteorological data
from this area limits our ability to explain this result as an effect of the changing ambient
temperature throughout the day.
The results of this study need to be considered in light of several limitations. First,
Venables and Mitchell (1996) suggest the importance of considering the interaction between
the time of the day and the season with sex, as females may be more responsive to these con-
ditions than men [56]. In addition to possible differences in physiological response between
the genders, different physical environments may also induce varying psychological re-
sponses. Park et al. (2011) studied the relationship between various physical environments
in forests and psychological responses; they found that they were significantly related [57].

Int. J. Environ. Res. Public Health 2022, 19, 1231
13 of 16
This study did not account for the differences between the genders, nor other physical
factors, apart from the average air temperature. Second, the participants were not required
to perform the various exercises in the Nature Break. For example, some participants did
not take off their shoes and socks to do the barefoot walking segment, which may have
confounded the current results. Third, the wearable sensor would occasionally disconnect
from the mobile application due to a Bluetooth malfunction. These intermittent disconnec-
tions accounted for several participants’ data being missing or too short to contribute to
the analysis. Furthermore, when these moments occurred, a researcher had to intervene
to check the equipment; this may have disrupted the complete immersive experience of
the Nature Break activity. Fourth, there may be an effect of physical activity on the phys-
iological responses in addition to the response from the forest bathing experience itself.
Olafsdottir et al. (2020) investigated the effects of leisure walking in a natural environment
against passive exposure to nature and physical exercise alone. They found that walking in
nature improved mood more than just watching nature or doing physical exercise alone.
They also found that, during a more stressful time period, walking in nature lowered
cortisol levels more than nature viewing [48]. Hansen et al. (2017) also mentioned that
multiple studies limited physical activities for each activity to 20 min in order to control for
the effect of physical exercise on cardiovascular measures [3]. Although in this study we
did not disentangle the effects of exercise on the physiological response from the effects
of forest bathing, results from previous studies indicate that the act of walking may have
contributed to the positive effects reported in our study.
Our study has shown that it is possible to use wearable sensors in order to monitor
forest bathing experiences and this has significant implications for all organizations that
aim to quantify the effect of nature on human physiology. Our results support using
technology for a more evidence-based approach to forest bathing. They set the stage for
future work that uses the distinct physiological response profiles that are associated with
specific forest activities as a scaffold to guide the design of a forest immersion experience
according to the intended outcome. Future work can also build upon the feasibility of using
wearable sensors for moment-by-moment physiological monitoring in the forest to detect
significant moments and activities for specific participants in real-time, allowing guides to
tailor and customize the forest experience to the individual forest bather.
5. Conclusions
We found that an interactive, guided nature activity increases positive mood states
and decreases negative mood states. The various exercises in the forest had significant and
varying physiological effects. In general, during the forest activity segments that involved
more external stimuli that required focus and attention, such as walking barefoot in the
forest or discussing personal experiences in the forest, the participants experienced a larger
sympathetic activation; whereas, during the segments of introspection and relaxation, the
participants experienced a larger parasympathetic activation. We found that there were
differences in the physiological responses due to circadian rhythm, however we are not
able to consistently report a main finding. For future forest bathing initiatives, we highlight
the possibility of creating a program that is shaped by a target physiological response.
Author Contributions: Conceptualization, E.T.M., H.M., S.B.-M.; methodology, E.T.M., H.M. and
S.B.-M.; data collection, D.F., N.I.S. and S.B.-M.; formal analysis, D.F. and S.B.-M.; writing—original
draft preparation, D.F.; writing—review and editing, D.F., N.I.S., S.B.-M.; supervision, S.B.-M.; project
administration, N.I.S., S.B.-M.; funding acquisition, S.B.-M. All authors have read and agreed to the
published version of the manuscript.
Funding: This study was funded by the Natural Sciences and Engineering Research Council (NSERC)
of Canada [Discovery Grant RGPIN-2016-03817; Engage Plus EGP2544529-19].
Institutional Review Board Statement: This study was approved by the Institutional Review Board
of McGill University (study number A10-M53-19A).

Int. J. Environ. Res. Public Health 2022, 19, 1231
14 of 16
Informed Consent Statement: Informed consent was obtained from all of the subjects who were
involved in the study.
Data Availability Statement: The physiological and POMS data that were used to support the
findings of this study are available from the corresponding author upon request.
Acknowledgments: The authors would like to thank R.M Ochoa, N. Kuhlmann for helping with
the data collection, J. Correa for his help with the statistical analysis, and M. Bouchard for his
technical support.
Conflicts of Interest: The authors declare no conflict of interest. The funders had no role in the design
of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or
in the decision to publish the results.
References
1. United Nations; Department of Economic and Social Affairs; Population Division. World Urbanization Prospects: The 2018 Revision;
United Nations: New York, NY, USA, 2019; ISBN 978-92-1-148319-2.
2. Vlahov, D. Urbanization, Urbanicity, and Health. J. Urban Health Bull. N. Y. Acad. Med. 2002, 79, S1–S12. [CrossRef] [PubMed]
3. Hansen, M.M.; Jones, R.; Tocchini, K. Shinrin-Yoku (Forest Bathing) and Nature Therapy: A State-of-the-Art Review. Int. J.
Environ. Res. Public Health 2017, 14, 851. [CrossRef]
4. Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The Physiological Effects of Shinrin-Yoku (Taking in the Forest
Atmosphere or Forest Bathing): Evidence from Field Experiments in 24 Forests across Japan. Environ. Health Prev. Med. 2009,
15, 18. [CrossRef] [PubMed]
5. Wen, Y.; Yan, Q.; Pan, Y.; Gu, X.; Liu, Y. Medical Empirical Research on Forest Bathing (Shinrin-Yoku): A Systematic Review.
Environ. Health Prev. Med. 2019, 24, 70. [CrossRef]
6. Kondo, M.C.; Jacoby, S.F.; South, E.C. Does Spending Time Outdoors Reduce Stress? A Review of Real-Time Stress Response to
Outdoor Environments. Health Place 2018, 51, 136–150. [CrossRef]
7. Oh, B.; Lee, K.J.; Zaslawski, C.; Yeung, A.; Rosenthal, D.; Larkey, L.; Back, M. Health and Well-Being Benefits of Spending Time in
Forests: Systematic Review. Environ. Health Prev. Med. 2017, 22, 71. [CrossRef] [PubMed]
8. Mao, G.-X.; Cao, Y.-B.; Lan, X.-G.; He, Z.-H.; Chen, Z.-M.; Wang, Y.-Z.; Hu, X.-L.; Lv, Y.-D.; Wang, G.-F.; Yan, J. Therapeutic Effect
of Forest Bathing on Human Hypertension in the Elderly. J. Cardiol. 2012, 60, 495–502. [CrossRef] [PubMed]
9. Hunter, M.R.; Gillespie, B.W.; Chen, S.Y.-P. Urban Nature Experiences Reduce Stress in the Context of Daily Life Based on Salivary
Biomarkers. Front. Psychol. 2019, 10, 722. [CrossRef] [PubMed]
10. Lee, J.; Park, B.-J.; Tsunetsugu, Y.; Ohira, T.; Kagawa, T.; Miyazaki, Y. Effect of Forest Bathing on Physiological and Psychological
Responses in Young Japanese Male Subjects. Public Health 2011, 125, 93–100. [CrossRef] [PubMed]
11. Bielinis, E.; Takayama, N.; Boiko, S.; Omelan, A.; Bielinis, L. The Effect of Winter Forest Bathing on Psychological Relaxation of
Young Polish Adults. Urban For. Urban Green. 2018, 29, 276–283. [CrossRef]
12. Horiuchi, M.; Endo, J.; Akatsuka, S.; Hasegawa, T.; Yamamoto, E.; Uno, T.; Kikuchi, S. An Effective Strategy to Reduce Blood
Pressure after Forest Walking in Middle-Aged and Aged People. J. Phys. Ther. Sci. 2015, 27, 3711–3716. [CrossRef]
13. Morita, E.; Fukuda, S.; Nagano, J.; Hamajima, N.; Yamamoto, H.; Iwai, Y.; Nakashima, T.; Ohira, H.; Shirakawa, T. Psychological
Effects of Forest Environments on Healthy Adults: Shinrin-Yoku (Forest-Air Bathing, Walking) as a Possible Method of Stress
Reduction. Public Health 2007, 121, 54–63. [CrossRef]
14. Mitten, D.; Overholt, J.R.; Haynes, F.I.; D’Amore, C.C.; Ady, J.C. Hiking. Am. J. Lifestyle Med. 2016, 12, 302–310. [CrossRef]
15. Kobayashi, H.; Song, C.; Ikei, H.; Kagawa, T.; Miyazaki, Y. Analysis of Individual Variations in Autonomic Responses to
Urban and Forest Environments . Available online: https://www.hindawi.com/journals/ecam/2015/671094/ (accessed on
29 January 2021).
16. Lee, M.; Lee, J.; Park, B.-J.; Miyazaki, Y. Interaction with Indoor Plants May Reduce Psychological and Physiological Stress by
Suppressing Autonomic Nervous System Activity in Young Adults: A Randomized Crossover Study. J. Physiol. Anthropol. 2015,
34, 21. [CrossRef] [PubMed]
17. Tsunetsugu, Y.; Park, B.-J.; Ishii, H.; Hirano, H.; Kagawa, T.; Miyazaki, Y. Physiological Effects of Shinrin-Yoku (Taking in the
Atmosphere of the Forest) in an Old-Growth Broadleaf Forest in Yamagata Prefecture, Japan. J. Physiol. Anthropol. 2007, 26,
135–142. [CrossRef] [PubMed]
18. Tsunetsugu, Y.; Park, B.-J.; Miyazaki, Y. Trends in Research Related to “Shinrin-Yoku” (Taking in the Forest Atmosphere or Forest
Bathing) in Japan. Environ. Health Prev. Med. 2010, 15, 27–37. [CrossRef] [PubMed]
19. Igarashi, M.; Miwa, M.; Ikei, H.; Song, C.; Takagaki, M.; Miyazaki, Y. Physiological and Psychological Effects of Viewing a
Kiwifruit (Actinidia Deliciosa ‘Hayward’) Orchard Landscape in Summer in Japan. Int. J. Environ. Res. Public. Health 2015, 12,
6657–6668. [CrossRef]
20. Jia, B.B.; Yang, Z.X.; Mao, G.X.; Lyu, Y.D.; Wen, X.L.; Xu, W.H.; Lyu, X.L.; Cao, Y.B.; Wang, G.F. Health Effect of Forest Bathing
Trip on Elderly Patients with Chronic Obstructive Pulmonary Disease. Biomed. Environ. Sci. BES 2016, 29, 212–218. [CrossRef]
[PubMed]

Int. J. Environ. Res. Public Health 2022, 19, 1231
15 of 16
21. Kobayashi, H.; Song, C.; Ikei, H.; Park, B.-J.; Lee, J.; Kagawa, T.; Miyazaki, Y. Population-Based Study on the Effect of a Forest
Environment on Salivary Cortisol Concentration. Int. J. Environ. Res. Public Health 2017, 14, 931. [CrossRef] [PubMed]
22. Im, S.G.; Choi, H.; Jeon, Y.-H.; Song, M.-K.; Kim, W.; Woo, J.-M. Comparison of Effect of Two-Hour Exposure to Forest and Urban
Environments on Cytokine, Anti-Oxidant, and Stress Levels in Young Adults. Int. J. Environ. Res. Public Health 2016, 13, 625.
[CrossRef] [PubMed]
23. Mao, G.X.; Cao, Y.B.; Yang, Y.; Chen, Z.M.; Dong, J.H.; Chen, S.S.; Wu, Q.; Lyu, X.L.; Jia, B.B.; Yan, J.; et al. Additive Benefits of
Twice Forest Bathing Trips in Elderly Patients with Chronic Heart Failure. Biomed. Environ. Sci. 2018, 31, 159–162. [CrossRef]
[PubMed]
24. Tsao, T.-M.; Tsai, M.-J.; Hwang, J.-S.; Cheng, W.-F.; Wu, C.-F.; Chou, C.-C.K.; Su, T.-C. Health Effects of a Forest Environment on
Natural Killer Cells in Humans: An Observational Pilot Study. Oncotarget 2018, 9, 16501–16511. [CrossRef]
25. Chen, Z.; Schulz, S.; Qiu, M.; Yang, W.; He, X.; Wang, Z.; Yang, L. Assessing Affective Experience of In-Situ Environmental Walk
via Wearable Biosensors for Evidence-Based Design. Cogn. Syst. Res. 2018, 52, 970–977. [CrossRef]
26. Elsadek, M.; Liu, B.; Lian, Z. Green Façades: Their Contribution to Stress Recovery and Well-Being in High-Density Cities. Urban
For. Urban Green. 2019, 46, 126446. [CrossRef]
27. Reeves, J.P.; Knight, A.T.; Strong, E.A.; Heng, V.; Neale, C.; Cromie, R.; Vercammen, A. The Application of Wearable Technology
to Quantify Health and Wellbeing Co-Benefits From Urban Wetlands. Front. Psychol. 2019, 10, 1840. [CrossRef] [PubMed]
28. Ulrich, R.S.; Simons, R.F.; Losito, B.D.; Fiorito, E.; Miles, M.A.; Zelson, M. Stress Recovery during Exposure to Natural and Urban
Environments. J. Environ. Psychol. 1991, 11, 201–230. [CrossRef]
29. Duvall, J. Enhancing the Benefits of Outdoor Walking with Cognitive Engagement Strategies. J. Environ. Psychol. 2011, 31, 27–35.
[CrossRef]
30. Duvall, J. Using Engagement-Based Strategies to Alter Perceptions of the Walking Environment. Environ. Behav. 2013, 45, 303–322.
[CrossRef]
31. Korpela, K.; Savonen, E.-M.; Anttila, S.; Pasanen, T.; Ratcliffe, E. Enhancing Wellbeing with Psychological Tasks along Forest
Trails. Urban For. Urban Green. 2017, 26, 25–30. [CrossRef]
32. Hartig, T.; Böök, A.; Garvill, J.; Olsson, T.; Gärling, T. Environmental Influences on Psychological Restoration. Scand. J. Psychol.
1996, 37, 378–393. [CrossRef] [PubMed]
33. Hassan, A.; Tao, J.; Li, G.; Jiang, M.; Aii, L.; Zhihui, J.; Zongfang, L.; Qibing, C. Effects of Walking in Bamboo Forest and City
Environments on Brainwave Activity in Young Adults. Available online: https://www.hindawi.com/journals/ecam/2018/965
3857/ (accessed on 14 January 2021).
34. Ochiai, H.; Ikei, H.; Song, C.; Kobayashi, M.; Miura, T.; Kagawa, T.; Li, Q.; Kumeda, S.; Imai, M.; Miyazaki, Y. Physiological
and Psychological Effects of a Forest Therapy Program on Middle-Aged Females. Int. J. Environ. Res. Public Health 2015, 12,
15222–15232. [CrossRef]
35. Ulrich, R.S. View through a Window May Influence Recovery from Surgery. Science 1984, 224, 420–421. [CrossRef] [PubMed]
36. Curran, S.L.; Andrykowski, M.A.; Studts, J.L. Short Form of the Profile of Mood States (POMS-SF): Psychometric Information.
Psychol. Assess. 1995, 7, 80–83. [CrossRef]
37. Cheung, S.; Han, E.; Kushki, A.; Anagnostou, E.; Biddiss, E. Biomusic: An Auditory Interface for Detecting Physiological
Indicators of Anxiety in Children. Front. Neurosci. 2016, 10, 401. [CrossRef] [PubMed]
38. Blain-Moraes, S.; Chau, T.; Mihailidis, A. Peripheral Autonomic Signals as Access Pathways for Individuals with Severe
Disabilities: A Literature Appraisal. Open Rehabil. J. 2008, 1, 27–37. [CrossRef]
39. Noyes, F.R.; Barber-Westin, S.D. 40-Diagnosis and Treatment of Complex Regional Pain Syndrome. In Noyes’ Knee Disorders:
Surgery, Rehabilitation, Clinical Outcomes, 2nd ed.; Noyes, F.R., Barber-Westin, S.D., Eds.; Elsevier: Amsterdam, The Netherlands,
2017; pp. 1122–1160. ISBN 978-0-323-32903-3.
40. Blain, S.; McKeever, P. Revealing Personhood through Biomusic of Individuals without Communicative Interaction Ability.
Augment. Altern. Commun. 2011, 27, 1–4. [CrossRef]
41. Shu, L.; Xie, J.; Yang, M.; Li, Z.; Li, Z.; Liao, D.; Xu, X.; Yang, X. A Review of Emotion Recognition Using Physiological Signals.
Sensors 2018, 18, 2074. [CrossRef] [PubMed]
42. Kistler, A.; Mariauzouls, C.; von Berlepsch, K. Fingertip Temperature as an Indicator for Sympathetic Responses. Int. J.
Psychophysiol. 1998, 29, 35–41. [CrossRef]
43. Kaushik, R.M.; Kaushik, R.; Mahajan, S.K.; Rajesh, V. Effects of Mental Relaxation and Slow Breathing in Essential Hypertension.
Complement. Ther. Med. 2006, 14, 120–126. [CrossRef]
44. Bruning, N.S.; Frew, D.R. Effects of Exercise, Relaxation, and Management Skills Training on Physiological Stress Indicators: A
Field Experiment. J. Appl. Psychol. 1987, 72, 515–521. [CrossRef] [PubMed]
45. Khanna, A.; Paul, M.; Sandhu, J.S. Efficacy of two relaxation techniques in reducing pulse rate among highly stressed females.
Calicut Med. J. 2007, 5, E3.
46. Lai Kwan, C.; Mahdid, Y.; Motta Ochoa, R.; Lee, K.; Park, M.; Blain-Moraes, S. Wearable Technology for Detecting Significant
Moments in Individuals with Dementia. BioMed Res. Int. 2019, 2019, e6515813. [CrossRef] [PubMed]
47. Blain, S.; Mihailidis, A.; Chau, T. Assessing the Potential of Electrodermal Activity as an Alternative Access Pathway. Med. Eng.
Phys. 2008, 30, 498–505. [CrossRef] [PubMed]

Int. J. Environ. Res. Public Health 2022, 19, 1231
16 of 16
48. Olafsdottir, G.; Cloke, P.; Schulz, A.; van Dyck, Z.; Eysteinsson, T.; Thorleifsdottir, B.; Vögele, C. Health Benefits of Walking in
Nature: A Randomized Controlled Study Under Conditions of Real-Life Stress. Environ. Behav. 2020, 52, 248–274. [CrossRef]
49. Song, C.; Ikei, H.; Kobayashi, M.; Miura, T.; Taue, M.; Kagawa, T.; Li, Q.; Kumeda, S.; Imai, M.; Miyazaki, Y. Effect of Forest
Walking on Autonomic Nervous System Activity in Middle-Aged Hypertensive Individuals: A Pilot Study. Int. J. Environ. Res.
Public Health 2015, 12, 2687–2699. [CrossRef]
50. Cox, D.T.C.; Hudson, H.L.; Shanahan, D.F.; Fuller, R.A.; Gaston, K.J. The Rarity of Direct Experiences of Nature in an Urban
Population. Landsc. Urban Plan. 2017, 160, 79–84. [CrossRef]
51. Cox, D.T.C.; Shanahan, D.F.; Hudson, H.L.; Plummer, K.E.; Siriwardena, G.M.; Fuller, R.A.; Anderson, K.; Hancock, S.; Gaston, K.J.
Doses of Neighborhood Nature: The Benefits for Mental Health of Living with Nature. BioScience 2017, 67, 147–155. [CrossRef]
52. Li, Q.; Kobayashi, M.; Kumeda, S.; Ochiai, T.; Miura, T.; Kagawa, T.; Imai, M.; Wang, Z.; Otsuka, T.; Kawada, T. Effects of Forest
Bathing on Cardiovascular and Metabolic Parameters in Middle-Aged Males. Evid. Based Complement. Alternat. Med. 2016,
2016, 2587381. [CrossRef]
53. Song, C.; Ikei, H.; Park, B.-J.; Lee, J.; Kagawa, T.; Miyazaki, Y. Psychological Benefits of Walking through Forest Areas. Int. J.
Environ. Res. Public Health 2018, 15, 2804. [CrossRef]
54. Williams, G.P.; Gold, L.W. Ground Temperatures. Can. Build. Dig. 1976, 6, 101. [CrossRef]
55. Korhonen, I. Blood Pressure and Heart Rate Responses in Men Exposed to Arm and Leg Cold Pressor Tests and Whole-Body
Cold Exposure. Int. J. Circumpolar Health 2006, 65, 178–184. [CrossRef]
56. Venables, P.H.; Mitchell, D.A. The Effects of Age, Sex and Time of Testing on Skin Conductance Activity. Biol. Psychol. 1996, 43,
87–101. [CrossRef]
57. Park, B.-J.; Furuya, K.; Kasetani, T.; Takayama, N.; Kagawa, T.; Miyazaki, Y. Relationship between Psychological Responses and
Physical Environments in Forest Settings. Landsc. Urban Plan. 2011, 102, 24–32. [CrossRef]
